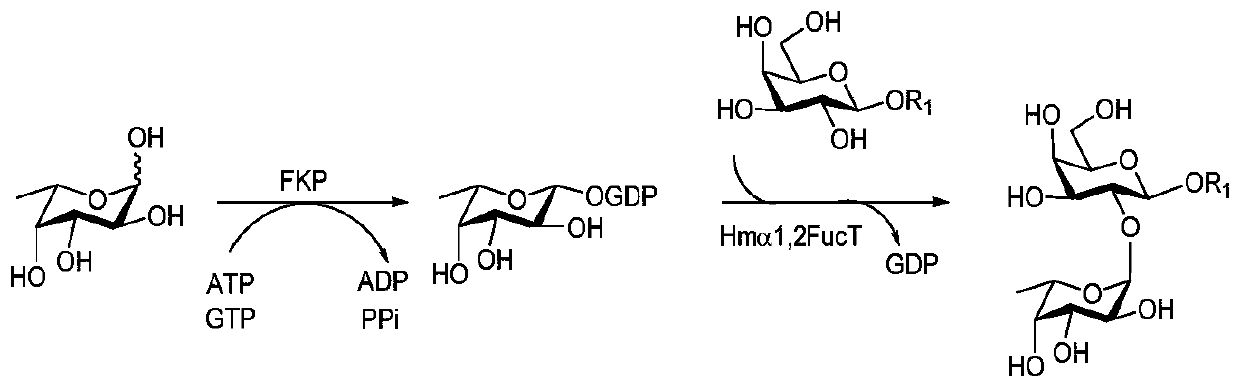
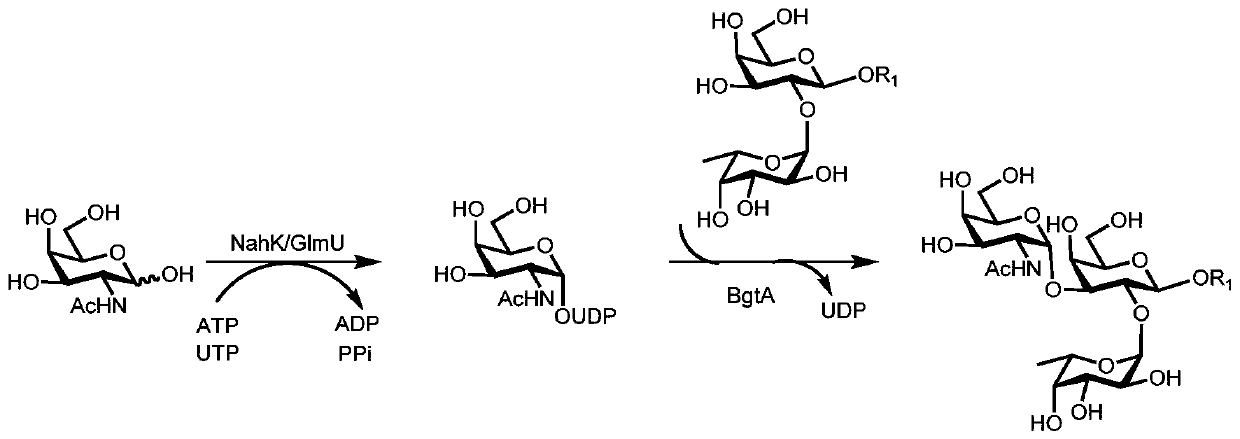
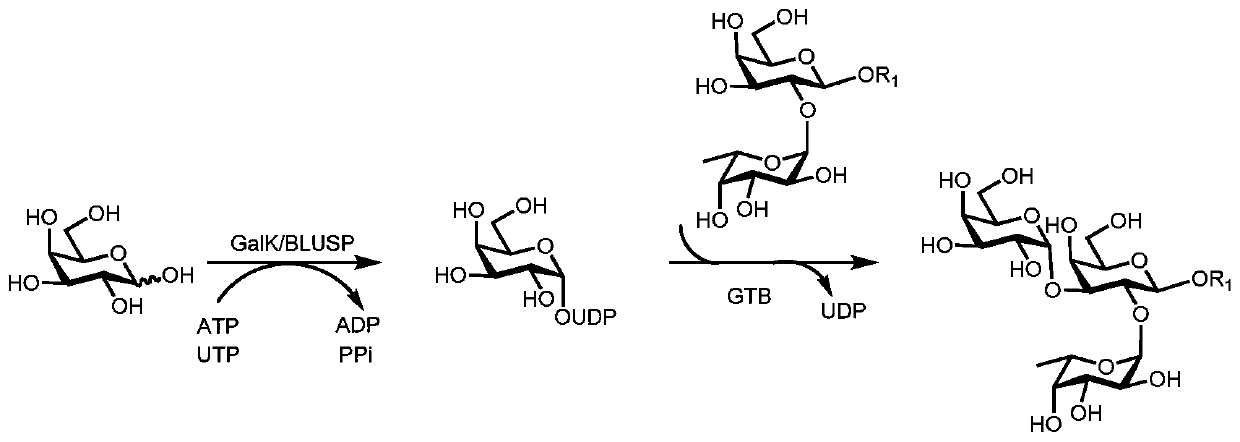
Synthesis method of GalNAc<alpha>1, 3Gal or Gal<alpha>1, 3Gal glycosidic bond oligosaccharide
A synthetic method and glycosidic bond technology, applied in biochemical equipment and methods, glycosyltransferase, transferase, etc., can solve problems such as limited application, enzyme instability, and difficulty in separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Synthesis of oligosaccharides containing GalNAcα1,3Gal or Galα1,3Gal glycosidic bonds
[0068] Proceed as follows:
[0069] (1) Enzymatic synthesis of disaccharide compound 1[Galβl,3GlcNAcβProN 3 ]
[0070] Compound 1 of the present invention [Galβl, 3GlcNAcβProN 3 ] The synthetic method is as follows:
[0071] Add receptor GlcNAcβProN to 50mL centrifuge tube 3 (0.10g, 0.33mmol), galactose (0.09g, 0.50mmol), adenosine triphosphate (ATP, 0.27g, 0.50mmol), Tris-HCl (100mmol, pH 7.5) and magnesium chloride (20mmol), dissolved In a little three-distilled water, shake until completely dissolved, adjust the pH to about 7.0 with 1mol / L HCl or 1mol / L NaOH, then add the enzyme GalK (2.00mg), BiGalHexNAcP (1.50mg), and finally decompose the reaction with three-distilled water The total volume of the liquid was added to 10mL, and reacted for 11h at 37°C under the condition of 100r / min, and the reaction progress was determined by thin-layer chromatography (EtOAc:MeO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com