Triptolide acrylate, and preparation method and application thereof
A technology of laurine ester and acrylic acid, applied in the field of medicine, can solve problems such as toxic and side effects, and achieve the effects of weakening toxicity, inhibiting cancer cell metastasis, and promoting apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention will be described in further detail below in conjunction with specific embodiments and accompanying drawings.
[0025]
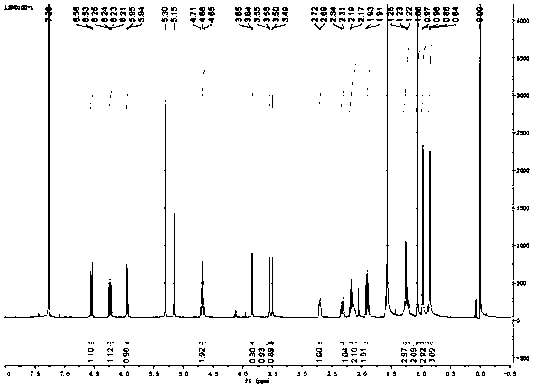
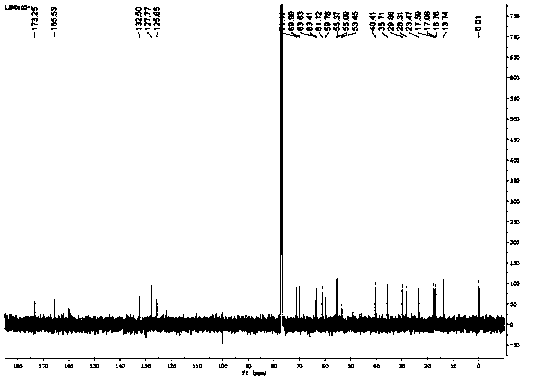
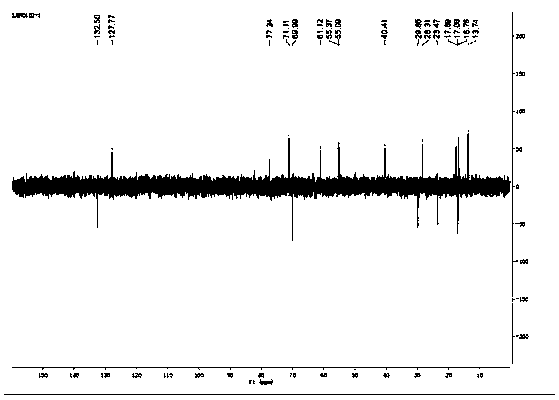
[0026] Take 103.4mg (0.287mmol) of triptolide and 1.75mg (0.01435mmol) of 4-dimethylaminopyridine and dissolve in 5mL of anhydrous dichloromethane, add 319.5mg (3.157mmol) of triethylamine, ice-bath to about 0°C, add 259.7 mg (2.87 mmol) of acryloyl chloride dropwise, gradually return to room temperature after dropping, stir for 2 hours, TLC detects that the reaction is complete, stop stirring, quench the reaction with saturated aqueous sodium bicarbonate, extract with dichloromethane, water The layer was extracted twice with dichloromethane, the dichloromethane extracts were combined, and most of the water in the dichloromethane extracts was washed away with saturated aqueous sodium chloride solution, then dried over anhydrous sodium sulfate, evaporated to dryness under reduced pressure, and prepared Separation on a thin-laye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com