Phosphate compound, and preparation method and application thereof
A technology of phosphate esters and compounds, which is applied in the field of phosphate ester compounds and their preparation, can solve the problems of low yield, low energy consumption, and large energy consumption, and achieve low waste discharge, high yield, and reaction equipment simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] A phosphate ester compound is 3,4-dimethoxyphenyl diphenylphosphinate, which has a molecular structure as shown in formula (II):
[0057] The preparation method of above-mentioned formula (II) compound, comprises the steps:
[0058] Take a 15mL pressure-resistant reaction tube, add 61mg of diphenylphosphine oxide, 231mg of 3,4-dimethoxyphenol, 36mg of chloroform, 69mg of 1,8-diazabicycloundec-7-ene (DBU), Acetonitrile 2mL, open reaction, stirred at room temperature for 3h. After the reaction was completed, 10 mL of ethyl acetate was added to quench the reaction, washed with 10 mL of saturated sodium sulfate solution, the organic phase was separated, the aqueous phase was extracted three times with ethyl acetate, the organic phases were combined, and separated by column chromatography to obtain 3,4-dimethyl Pure oxyphenyl diphenyl phosphinate (colorless oil) 101 mg, yield 95%; wherein the molar ratio of diphenyl phosphine oxide to 3,4-dimethoxyphenol is 1:5, The mola...
Embodiment 2
[0068] A phosphate ester compound is 2,3-dihydroxypropyl diphenylphosphinate, which has a molecular structure as shown in formula (III):
[0069] The preparation method of the above-mentioned compound of formula (III) is the same as in Example 1, except that 231 mg of 3,4-dimethoxyphenol is replaced by 138 mg of glycerol to obtain 2,3-dihydroxypropyldiphenylene 85 mg of pure phosphonate, yield 96%; wherein the molar ratio of diphenylphosphine oxide to glycerin is 1:5, the molar ratio of diphenylphosphine oxide to chloroform is 1:1, and the molar ratio of diphenylphosphine oxide to chloroform is 1:1. The DBU molar ratio is 1:1.5.
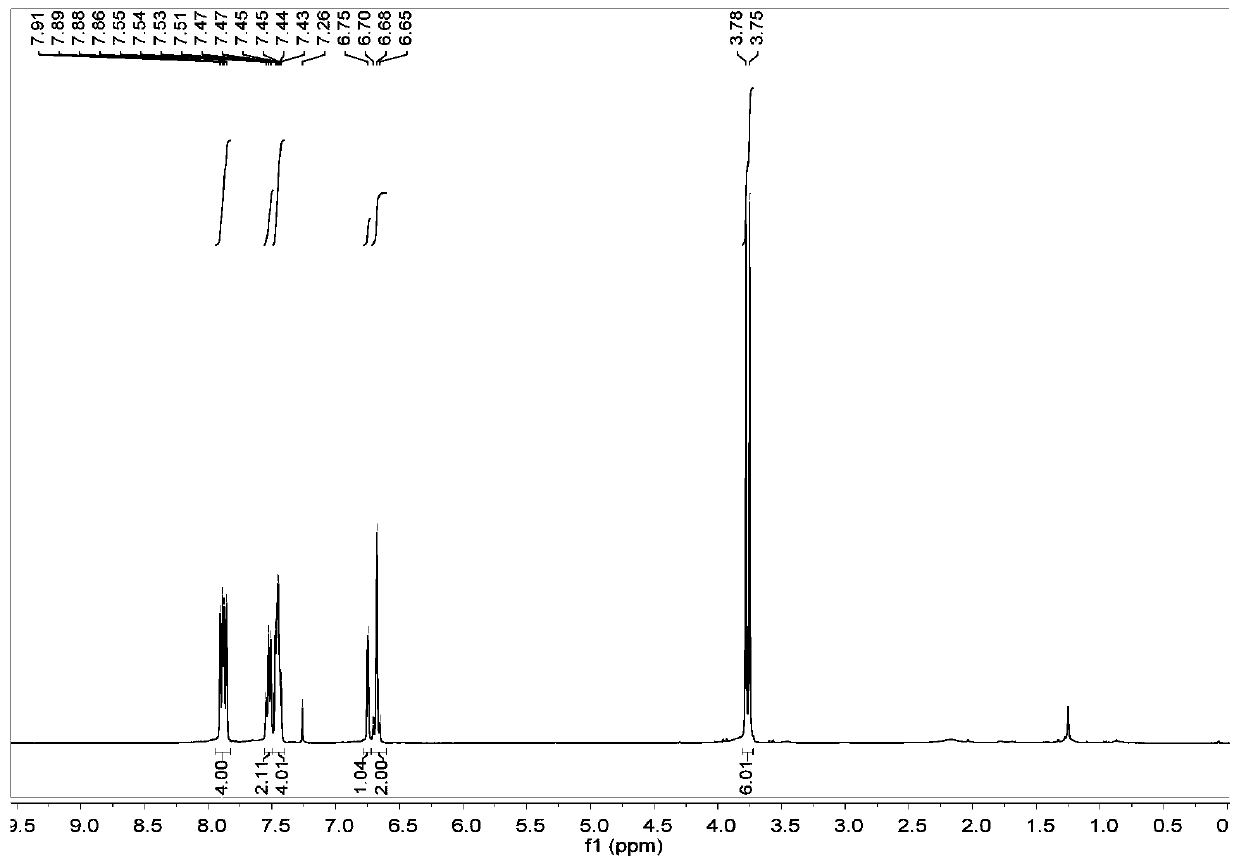
[0070] The NMR results of the above-mentioned formula (Ⅲ) compound are as follows: Figure 4 , 1 H NMR (400MHz, CDCl 3 )δ7.85–7.75(m,4H),7.60–7.51(m,2H),7.50–7.41(m,4H),4.11(dd,J=11.0,5.0Hz,2H),3.95(p,J= 4.8Hz, 1H), 3.71(d, J=4.7Hz, 2H).
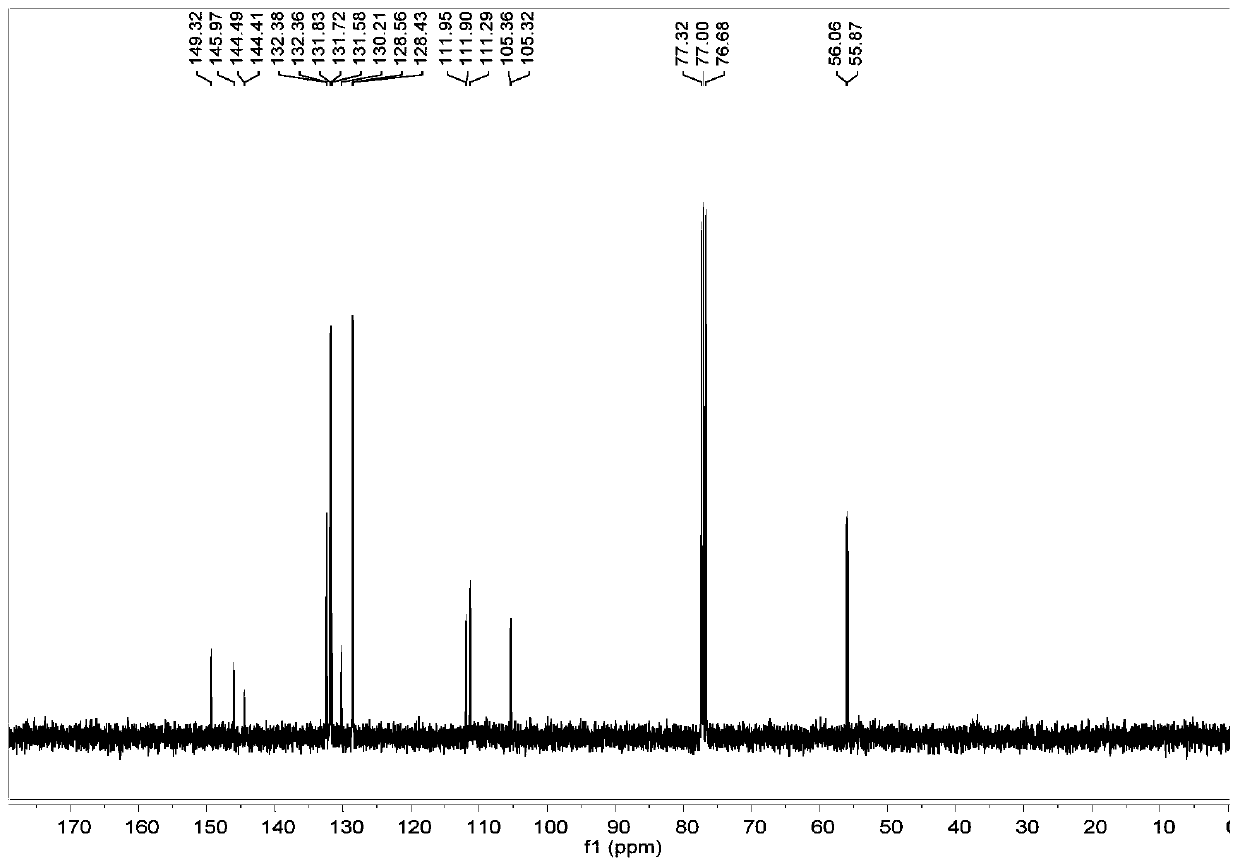
[0071] From Figure 5 It can be seen that 13 C NMR (101MHz, CDCl 3 )δ132.95–132.27(m), 131.67(dd, J=10...
Embodiment 3
[0077] A phosphate compound is methyl bis[[1,1'-biphenyl]-4-yl]phosphinate, and the phosphate compound has a molecular structure as shown in formula (IV):
[0078] The preparation method of the above-mentioned compound of formula (IV) is the same as that of Example 1, except that diphenylphosphine oxide is replaced by 106 mg of bis([1,1'-biphenyl]-4-yl)phosphine oxide, 3,4- Dimethoxyphenol was replaced by methanol 48mg, and acetonitrile was replaced by polyethylene glycol 200 2mL to obtain 98mg of pure methyl bis[[1,1'-biphenyl]-4-yl]phosphinate (white solid), The yield is 85%; the molar ratio of bis([1,1'-biphenyl]-4-yl)phosphine oxide to methanol is 1:5, and bis([1,1'-biphenyl]-4-yl) The molar ratio of phosphine oxide to chloroform is 1:1, and the molar ratio of bis([1,1'-biphenyl]-4-yl)phosphine oxide to DBU is 1:1.5.
[0079] The NMR results of the above-mentioned formula (VII) compound are as follows: Figure 7 , 1 H NMR (400MHz, CDCl 3 )δ7.94(dd, J=11.9,8.3Hz,4H),7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com