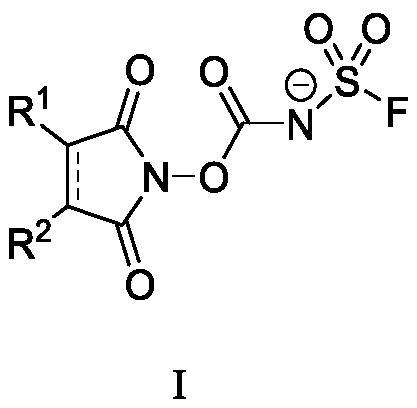
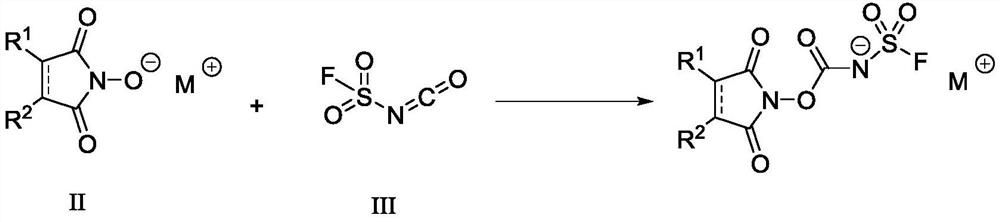
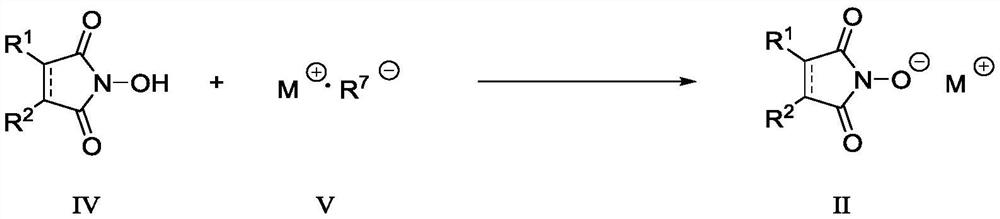N-substituted carbonyl fluorine sulfonamide compound as well as preparation method and application thereof
A carbonyl fluorosulfonamide and compound technology, which is applied in the preparation of sulfuric acid amide, organic chemistry, etc., can solve the problems of increased storage and use, no commercialization, and easy volatility, and achieves low toxicity, simple preparation, and wide substrate adaptability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0193]
[0194] Dried N-hydroxysuccinimide (1.15g, 10mmol) was added to 10mL of ultra-dry methanol, stirred at 0°C, sodium methoxide (540mg, 10mmol) was added, and returned to room temperature. After two hours of reaction, the solvent was spin-dried, and the water was removed by azeotropic evaporation with toluene three times, and the residual solvent was drained by an oil pump. Add 20 mL of ultra-dry acetonitrile, stir at 0°C, add dropwise fluorosulfonyl isocyanate (0.8 mL, 10 mmol), return to room temperature, and react for 2 hours. Concentrate the solvent to about 5 mL, and add dropwise to 80 mL of vigorously stirred methyl A solid was precipitated from tert-butyl ether, filtered, washed with 5 mL of methyl tert-butyl ether, and the residual solvent was drained by an oil pump to obtain 2.2 g of a white solid with a yield of 85% and a decomposition temperature of 163.2°C. 1 H NMR (400MHz, CD 3 CN)δ2.70(s,4H); 13 C NMR (101MHz, CD 3 CN) δ172.1, 155.5, 26.2; 19 F NMR (3...
Embodiment 2
[0196]
[0197] Add dried N-hydroxysuccinimide (11.5 g, 100 mmol) into 100 mL of ultra-dry methanol, stir at 0°C, add potassium tert-butoxide (11.2 g, 100 mmol), and return to room temperature. After two hours of reaction, the solvent was spin-dried, and the water was removed by azeotropic evaporation with toluene three times, and the residual solvent was drained by an oil pump. Add 200mL of ultra-dry acetonitrile, stir at 0°C, add dropwise fluorosulfonyl isocyanate (8mL, 100mmol), return to room temperature, and react for 2h, concentrate the solvent to about 50mL, and add dropwise to 800mL of vigorously stirred methyl tert-methyl A solid was precipitated from butyl ether, filtered, washed with 50 mL of methyl tert-butyl ether, and the residual solvent was drained by an oil pump to obtain 24.5 g of a white solid with a yield of 87% and a decomposition temperature of 166.7°C. 1 H NMR (400MHz, DMSO) δ2.71 (s, 4H); 13 C NMR (101MHz, DMSO) δ171.2, 153.3, 25.3; 19 F NMR (376MH...
Embodiment 3
[0199]
[0200] Dried N-hydroxysuccinimide (391mg, 3.4mmol) was added to 5mL of ultra-dry methanol, stirred at 0°C, and tetramethylammonium hydroxide (wt.25% in methanol) (1.22g, 3.4mmol), returned to room temperature. After two hours of reaction, the solvent was spin-dried, and the water was removed by azeotropic evaporation with toluene three times, and the residual solvent was drained by an oil pump. Add 10 mL of ultra-dry acetonitrile, stir at 0°C, add dropwise fluorosulfonyl isocyanate (0.27 mL, 3.4 mmol), return to room temperature, and react for 2 hours. Concentrate the solvent to about 1 mL, and add dropwise to 10 mL of vigorously stirred formazan with a dropper. A solid was precipitated from methyl tert-butyl ether, filtered, washed with 3 mL of methyl tert-butyl ether, and the residual solvent was drained by an oil pump to obtain 1.7 g of a white solid with a yield of 80% and a decomposition temperature of 155.6°C. 1 H NMR (400MHz, CD 3 CN) δ3.08(s,12H),2.70(s,4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com