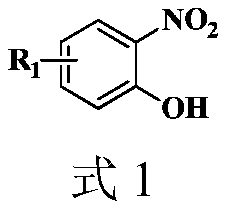
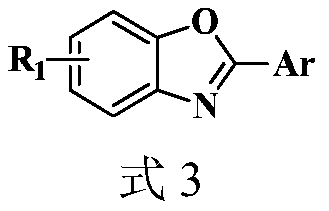
Synthesis method of 2-aryl benzoxazole derivative
A benzoxazole and synthetic method technology, applied in the field of heterocyclic compound synthesis, can solve the problems of low reaction efficiency, difficulty in obtaining the use cost of accelerators, etc., and achieve high reaction yield, low cost, and wide substrate adaptability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
[0059] Following examples 1~5 all react by following reaction equation:
[0060]
[0061] The specific operation steps are: in a 50mL round bottom flask, add o-nitrophenol derivatives (10mmol), benzaldehyde derivatives (10mmol), sulfur powder (20mmol), pyridine (10mmol) in sequence, and the resulting mixture is placed in a 60°C water bath In the process, the ultrasonic frequency is 68KHz, and the ultrasonic reaction is 40 minutes under the condition of power 60W. After the reaction, dissolve the reactant in 40 mL of ethyl acetate, wash the reactant with dilute hydrochloric acid solution, extract with 30 mL of ethyl acetate, combine the organic phases, concentrate the extract under reduced pressure, and dry in vacuum to calculate the weight.
[0062] The reaction product and corresponding yield obtained in the following examples are as follows:
[0063]
Embodiment 1
[0065] 2-phenylbenzo[d]oxazole(A)
[0066] 1 H NMR (400MHz, CDCl 3 ):δ8.27–8.25(m,2H),7.81–7.76(m,1H),7.60–7.57(m,1H),7.55–7.53(m,3H),7.39–7.35(m,2H);
[0067] 13 C NMR (100MHz, CDCl 3 ): δ163.3, 150.9, 142.3, 131.8, 129.1, 127.8, 127.4, 1254, 124.6, 120.2, 110.8.
Embodiment 2
[0069] 5-methyl-2-phenylbenzo[d]oxazole(B)
[0070] 1 H NMR (400MHz, CDCl 3 ):δ8.27–8.24(m,2H),7.55(s,1H),7.54-7.52(m,3H),7.46(d,J=8.4Hz,1H); 7.17(d,J=8.4Hz, 1H),2.48(s,3H);
[0071] 13 C NMR (100MHz, CDCl 3 ): δ163.2, 149.1, 142.5, 134.4, 131.4, 129.0, 127.8, 127.5, 126.4, 120.1, 110.0, 21.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com