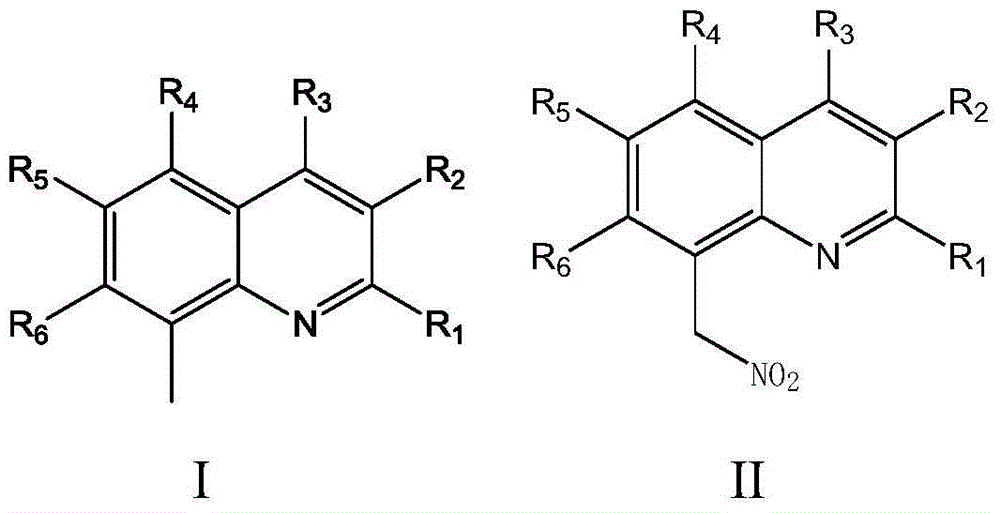
Method for synthesizing 8-(nitro methyl) quinoline compounds
A technology of methylquinoline and nitromethyl, which is applied in the field of synthesis of organic compounds, can solve the problems of affecting the reaction, difficult nitration reaction, low product yield and purity, and achieves simple reaction steps, good selectivity, Good substrate adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
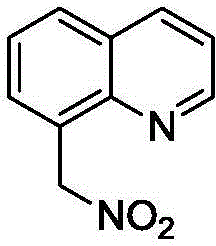
[0032] (1) 0.5 mmol (71.5 mg) of 8-methylquinoline, 0.05 mmol (11.2 mg) of palladium diacetate, 0.75 mmol (100.1 mg) of N-chlorosuccinimide, and 0.75 mmol (115.4 mg) of silver nitrite mg) and 5ml of acetonitrile were sequentially added into a sealed pressure vessel with a volume of 25ml. The mixture was heated in an oil bath at 90°C for 48 hours. After the TLC detection reaction finished, the reaction solution was diluted with dichloromethane, filtered to obtain the clear liquid, separated by column chromatography (eluent ratio: sherwood oil to ethyl acetate volume ratio 10:1), and collected the product containing Eluate, eluate was evaporated to obtain 8-(nitromethyl)quinoline (93% yield).
[0033]
[0034] White solid; m.p.64-65°C; IR(KBr):ν=1518(NO 2 )cm -1 ; 1 H NMR (CDCl 3 ,500MHz):δ8.98(dd,J 1 =4.5,J 2 =2.0Hz,1H),8.20(dd,J 1 =8.5,J 2 =2.0Hz,1H),7.89(dd,J 1 =8.5Hz,J 2 =1.0Hz, 1H), 7.81(d, J=7.0Hz, 1H), 7.57(t, J=8.5Hz, 1H), 7.48(dd, J 1 =8.0Hz,J 2 =4.5Hz, ...
Embodiment 2
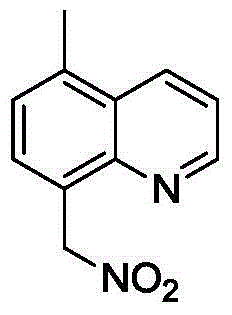
[0036] 0.5 mmol of 5,8-dimethylquinoline, 0.05 mmol of palladium dichloride, 1 mmol of N-chlorosuccinimide, 1 mmol of silver nitrite and 4 ml of acetonitrile were sequentially added into a 25 ml sealed pressure vessel. The mixture was heated and reacted in an oil bath at 100° C. for 48 hours. After the TLC detection reaction finished, the reaction solution was diluted with dichloromethane, filtered to obtain the clear liquid, separated by column chromatography (eluent ratio: sherwood oil to ethyl acetate volume ratio 10:1), and collected the product containing Eluate, eluate was evaporated to give 5-methyl-8-(nitromethyl)quinoline (43% yield).
[0037]
[0038] White solid, m.p.82-84℃; IR(neat):ν=1515(NO 2 )cm -1 ; 1 H NMR (CDCl 3 ,500MHz):δ8.98(dd,J 1 =4.5Hz,J 2=1.5Hz,1H),8.38(dd,J 1 =8.5Hz,J 2 =2.0Hz,1H),7.70(d,J=9.0Hz,1H),7.50(dd,J 1 =8.5Hz,J 2 =4.0Hz, 1H), 7.40(d, J=7.0Hz, 1H), 6.17(s, 2H), 2.72(s, 3H); 13 C NMR (CDCl 3 ,125MHz): δ149.8,146.3,136.8,132.9,130...
Embodiment 3
[0040] 0.5 mmol of 6,8-dimethylquinoline, 0.05 mmol of palladium trifluoroacetate, 0.75 mmol of N-chlorosuccinimide, 0.75 mmol of silver nitrite and 5 ml of acetonitrile were sequentially added into a 25 ml sealed pressure vessel. The mixture was heated and reacted in an 80° C. oil bath for 30 hours. After the TLC detection reaction finished, the reaction solution was diluted with dichloromethane, filtered to obtain the clear liquid, separated by column chromatography (eluent ratio: sherwood oil to ethyl acetate volume ratio 10:1), and collected the product containing Eluate, eluate was evaporated to give 6-methyl 8-(nitromethyl)quinoline (53% yield).
[0041]
[0042] White solid; m.p.62-64°C; IR(KBr):ν=1517(NO 2 )cm -1 ; 1 H NMR (CDCl 3 ,500MHz): δ8.90(d,J=2.5Hz,1H),8.11(dd,J 1 =8.0Hz,J 2 =1.5Hz,1H),7.64(s,2H),7.44(dd,J 1 =8.0Hz,J 2 =4.0Hz, 1H), 6.18(s, 2H), 2.56(s, 3H); 13 C NMR (CDCl 3 ,125MHz): δ149.5,144.8,136.1,135.6,132.2,130.2,128.4,128.2,71.2,21.6;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com