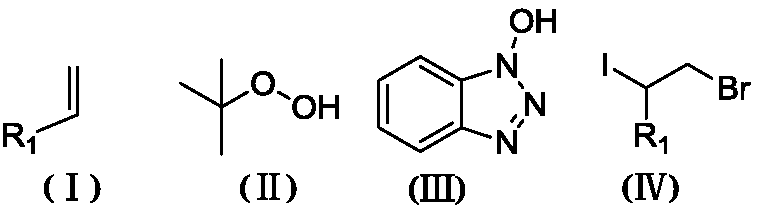
Method for synthesizing 2-bromo-1-iododihalide by one-pot process
A synthesis method and a dihalogenation technology are applied in the field of synthesizing 2-bromo-1-iodine dihalide, can solve problems such as difficult operation, environmental pollution, limitation of large-scale application, etc., and achieve simple reaction operation, mild reaction conditions, Good substrate adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Styrene (114.9 μL, 1 mmol), elemental iodine (26.9 mg, 0.5 mmol), 70% tert-butyl hydroperoxide (257.5 mg, 2 mmol) and N-hydroxybenzotriazole (166.6 mg, 1.2 mmol) were mixed Add 3ml of dichloromethane solvent into the flask and react at 40°C for 4h. After the reaction was detected by TLC, it was lowered to room temperature, and acetyl bromide (202.2 μL, 5 mmol) was added to react for 16 hours. After the reaction was detected by TLC, it was separated by column chromatography (eluent: petroleum ether) to obtain (2-bromo-1 -Iodoethyl)benzene (compound 1) 117.7 mg, yield 56.2%.
[0030] Product characterization: white solid, m.p. 60°C. 1 H NMR (500MHz, CDCl 3 )δ7.39 (tdd, J=8.6, 5.6, 3.2Hz, 5H), 5.15 (dd, J=10.7, 5.4Hz, 1H), 4.12–4.00 (m, 2H). 13 C NMR (125MHz, CDCl 3 )δ138.63, 129.20, 128.88, 127.67, 50.89, 35.03. HRMS (ESI) calcd for C 8 h 8 BrI(M+H + ):310.96, found 309.89.
Embodiment 2
[0032]
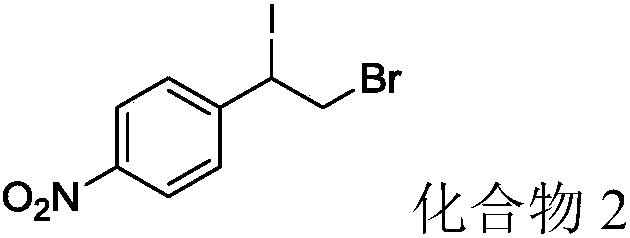
[0033] P-nitrostyrene (44.7mg, 0.3mmol), elemental iodine (38.1mg, 0.15mmol), 70% tert-butyl hydroperoxide (77.25mg, 0.6mmol) and N-hydroxybenzotriazole (49.1 mg, 0.36mmol) were mixed with 1ml of dichloromethane solvent into the flask and reacted at 40°C for 6h. After the reaction was detected by TLC, it was lowered to room temperature, and acetyl bromide (121.33 μL, 1.5 mmol) was added to react for 16 hours. After the reaction was detected by TLC, 1-(2- Bromo-1-iodoethyl)-4-nitrobenzene (compound 2) 68.6 mg, yield 64.2%.
[0034] Product characterization: Pale yellow solid, m.p.79°C. 1 H NMR (500MHz, CDCl 3 )δ 8.30–8.22(m,2H),7.62–7.56(m,2H),5.17(dd,J=11.3,4.9Hz,1H), 4.13–3.94(m,2H). 13 C NMR (125MHz, CDCl 3 )δ148.06, 145.50, 128.83, 124.12, 47.78, 33.88. HRMS (ESI) calcd for C 8 h 7 BRINO 2 (M+H + ):355.96, found 354.87.
Embodiment 3
[0036]
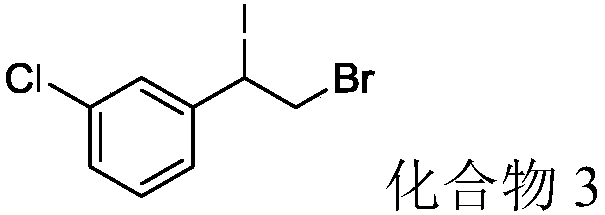
[0037]3-chlorostyrene (43.3mg, 0.3mmol), elemental iodine (38.1mg, 0.15mmol), 70% tert-butyl hydroperoxide (77.25mg, 0.6mmol) and N-hydroxybenzotriazole (49.1 mg, 0.36mmol) was mixed with 1ml of dichloromethane solvent into the flask and reacted at 40°C for 4h. After the reaction was detected by TLC, it was lowered to room temperature, and acetyl bromide (121.3 μL, 1.5 mmol) was added to react for 16 hours. After the reaction was detected by TLC, 1-chloro-3 was separated by column chromatography (eluent: petroleum ether). -(1-bromo-2-iodoethyl)benzene (compound 3) 68.1 mg, yield 65.7%.
[0038] Product characterization: white solid, m.p.95°C. 1 H NMR (500MHz, CDCl 3 )δ7.40 (d, J = 0.9Hz, 1H), 7.33 (dd, J = 3.8, 1.7Hz, 2H), 7.29 (ddd, J = 7.4, 4.5, 1.3Hz, 1H), 5.07 (dd, J =11.0,5.1Hz,1H),4.08–3.94(m,2H). 13 C NMR (126MHz, CDCl 3 )δ140.56, 134.68, 130.10, 129.36, 127.91, 125.91, 49.30, 34.56. HRMS (ESI) calcd for C 8 h 7 BrClI (M+H + ):345.40, found343.85.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com