Method for synthesizing polysubstituted dihydrofuran
A technology of dihydrofuran and synthesis method, which is applied in the field of preparation of multi-substituted dihydrofuran, can solve problems such as low utilization rate of atoms, complex operation conditions, expensive metal catalysts, etc., and achieve cheap and environmentally friendly reagents, high atom economy, Good substrate adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
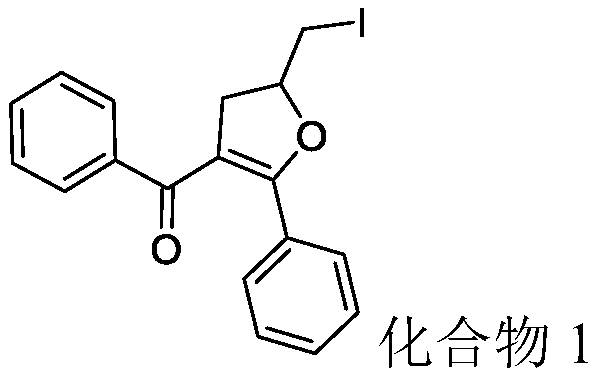
[0030] 2-allyl-1,3-diphenyl-1,3-propanedione (52.8mg, 0.2mmol), elemental iodine (25.4mg, 0.1mmol) and tert-butyl hydroperoxide (51.4mg, 0.4mmol, 70% aqueous solution) was added to the reaction flask, and finally 2ml of acetonitrile was added, and then reacted at room temperature for 12h. After the reaction, the compound 1 (56.7 mg) was separated by column chromatography (eluent: petroleum ether / ethyl acetate volume ratio 50:1), and the yield was 72.7%.
[0031] Product characterization: white solid; m.p.104-106°C; 1 H NMR (500MHz, CDCl 3 )δ / ppm=7.46-7.44(dd, J=1.2Hz, 8.4Hz, 2H), 7.24-7.16(m, 4H), 7.09-7.04(dd, J=7.5Hz, 15Hz, 2H), 4.90-4.85 (m,1H),3.53-3.46(m,3H),3.10-3.05(dd,J=7Hz,15.4Hz,1H).
Embodiment 2
[0033]
[0034] 2-allyl-1,3-bis(4-methoxyphenyl)-1,3-propanedione (64.8mg, 0.2mmol), elemental iodine (25.4mg, 0.1mmol) and tert-butyl Hydrogen peroxide (51.4mg, 0.4mmol, 70% aqueous solution) was added to the reaction flask, and finally 2ml of acetonitrile was added, and then reacted at room temperature for 12h. After the reaction, the compound 2 (65.9 mg) was separated by column chromatography (eluent: petroleum ether / ethyl acetate volume ratio 20:1), and the yield was 73.2%.
[0035] Product characterization: colorless oily liquid; 1 H NMR (500MHz, CDCl 3 )δ=7.53-7.51(dt, J=2Hz, 8.9Hz, 2H), 7.23-7.21(dt, J=2Hz, 8.9Hz, 2H), 6.65-6.61(m, 4H), 4.87-4.81(m, 1H),3.74(s,3H),3.72(s,3H),3.48-3.46(d,J=5.8Hz,2H),3.45-3.43(t,J=5.3Hz,10Hz,1H),3.06-3.01 (dd,J=7Hz,15.4Hz,1H).
Embodiment 3
[0037]
[0038] 2-allyl-1,3-bis(4-methylphenyl)-1,3-propanedione (58.4mg, 0.2mmol), elemental iodine (25.4mg, 0.1mmol) and tert-butyl per Hydrogen oxide (51.4mg, 0.4mmol, 70% aqueous solution) was added to the reaction flask, and finally 2ml of acetonitrile was added, and then reacted at room temperature for 12h. After the reaction, the compound 3 (58.6 mg) was separated by column chromatography (eluent: petroleum ether / ethyl acetate volume ratio 20:1), and the yield was 70.1%.
[0039] Product characterization: colorless oily liquid; 1 H NMR (500MHz, CDCl3 )δ=7.41-7.39(d, J=8.2Hz, 2H), 7.14-7.12(d, J=8.2Hz, 2H), 6.92-6.88(m, 4H), 4.88-4.82(m, 1H), 3.48 -3.47(d, J=5.8Hz, 2H), 3.46-3.43(t, J=5.3Hz, 10Hz, 1H), 3.07-3.03(dd, J=7Hz, 15.4Hz, 1H), 2.24(s, 3H ),2.23(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com