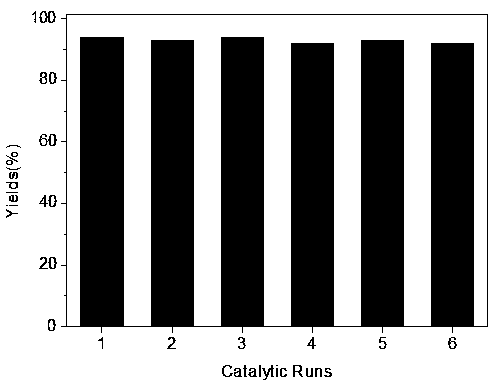
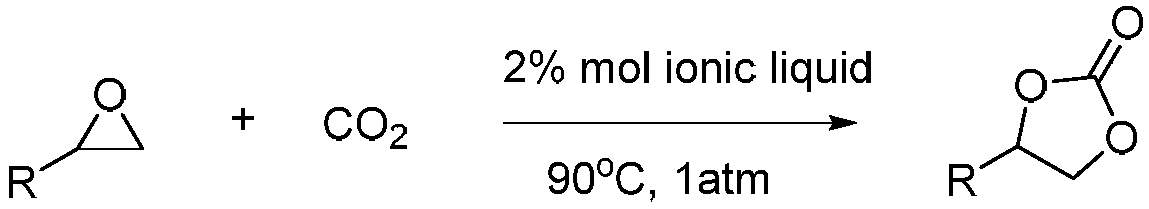Method of catalyzing conversion of CO2 with alcohol amine bromide ionic liquid to synthesize cyclic carbonate compound
A technology for cyclic carbonates and ionic liquids, which is applied in the field of ionic liquids catalyzing the synthesis of cyclic carbonates, can solve the problems of energy consumption, increased cost and danger of high-pressure equipment, and achieves easy preparation and good biological properties. The effect of simple capacitance, operation and post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] In a dry 50mL round-bottomed flask, fully dissolve 2 mol of N,N-dimethylethanolamine in 20mL of ethanol, and slowly add bromine in an equimolar amount to N,N-dimethylethanolamine using a constant pressure dropping funnel under stirring. Substitute n-butane in ethanol, heat to reflux at 80°C and stir for 13h. After the reaction, a large amount of white solids precipitated in the bottle, and the solvent was removed by suction filtration under reduced pressure. The solid was recrystallized three times with ether and ethyl acetate, filtered under reduced pressure, and the solid product was dried in a vacuum oven at 40°C for 48 hours to obtain Pure target product C 4 DMEABr.
Embodiment 2
[0019] In a dry 50mL round-bottomed flask, fully dissolve 2 mol of N,N-dimethylethanolamine in 20mL of ethanol, and slowly add bromine in an equimolar amount to N,N-dimethylethanolamine using a constant pressure dropping funnel under stirring. Substitute n-hexane ethanol solution, heat to reflux reaction at 80°C, and stir for 13h. After the reaction, a large amount of white solids precipitated in the bottle, and the solvent was removed by suction filtration under reduced pressure. The solid was recrystallized three times with ether and ethyl acetate, filtered under reduced pressure, and the solid product was dried in a vacuum oven at 40°C for 48 hours to obtain Pure target product C 6 DMEABr.
Embodiment 3
[0021] In a dry 50mL round-bottomed flask, fully dissolve 2 mol of N,N-dimethylethanolamine in 20mL of ethanol, and slowly add bromine in an equimolar amount to N,N-dimethylethanolamine using a constant pressure dropping funnel under stirring. On behalf of n-octane ethanol solution, heated to reflux reaction at 80 ° C, stirred for 13h. After the reaction, a large amount of white solids precipitated in the bottle, and the solvent was removed by suction filtration under reduced pressure. The solid was recrystallized three times with ether and ethyl acetate, filtered under reduced pressure, and the solid product was dried in a vacuum oven at 40°C for 48 hours to obtain Pure target product C 8 DMEABr.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com