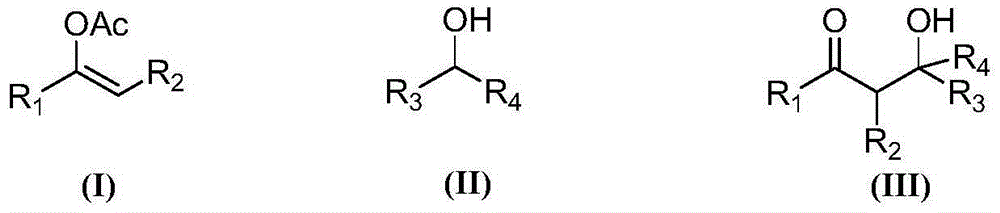
Synthesis method for beta-hydroxy-ketone compound
A synthetic method and compound technology, applied in the field of synthesizing β-hydroxy ketone compounds, can solve the problems of unfavorable practical application and restriction of wide application, and achieve the effects of good substrate adaptability, simple reaction operation, and avoiding residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
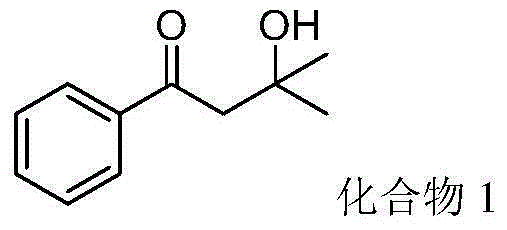
[0035] 1-Phenylvinyl acetate (81mg, 0.5mmol), isopropanol (2mL, 26.2mmol) and tert-butyl hydroperoxide (257.2mg, 2.0mmol, 70% aqueous solution) were added to the flask, at 100 °C for 10 hours. After the reaction was detected by TLC, it was separated by column chromatography (eluent: petroleum ether / ethyl acetate volume ratio 6:1) to obtain 3-hydroxyl-3-methyl-1-phenylbutan-1-one (Compound 1) 56.1 mg, yield 63%.
[0036] Product characterization: pale yellow liquid; 1 H NMR (500MHz, CDCl 3 )δ7.96(dt,J=8.5,1.5Hz,2H),7.63–7.56(m,1H),7.51–7.46(m,2H),3.16(s,2H),1.35(s,6H).13C NMR (125MHz, CDCl 3 )δ200.7, 136.5, 132.5, 127.7, 127.1, 68.9, 47.7, 28.6.
Embodiment 2
[0038]
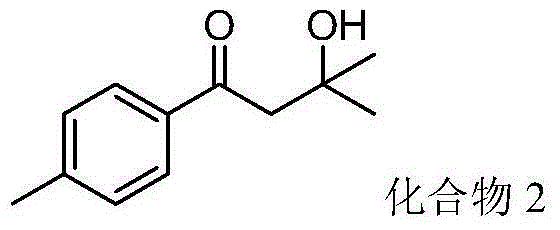
[0039] 1-(p-Methylphenyl) vinyl acetate (88 mg, 0.5 mmol), isopropanol (2 mL, 26.2 mmol) and tert-butyl hydroperoxide (257.2 mg, 2.0 mmol, 70% aqueous solution) were added to In the flask, the reaction was carried out at 100° C. for 12 hours. After the reaction was detected by TLC, it was separated by column chromatography (eluent: petroleum ether / ethyl acetate volume ratio 6:1) to obtain 3-hydroxyl-3-methyl-1-p-methylphenylbutane- 1-ketone (compound 2) 51 mg, yield 53%.
[0040] Product characterization: pale yellow liquid; 1 H NMR (500MHz, CDCl 3 )δ7.85(d, J=8.2Hz, 2H), 7.27(d, J=8.6Hz, 2H), 3.12(s, 2H), 2.42(s, 3H), 1.34(s, 6H). 13 C NMR (125MHz, CDCl 3 )δ201.5, 144.6, 134.8, 129.4, 128.2, 69.9, 48.4, 29.6, 21.7.
Embodiment 3
[0042]
[0043] 1-(4-Chlorophenyl)vinyl acetate (98 mg, 0.5 mmol), isopropanol (2 mL, 26.2 mmol) and tert-butyl hydroperoxide (257.2 mg, 2.0 mmol, 70% aqueous solution) were added to In the flask, the reaction was carried out at 100° C. for 6 hours. After the reaction was detected by TLC, it was separated by column chromatography (eluent: petroleum ether / ethyl acetate volume ratio 6:1) to obtain 3-hydroxyl-1-(3-methoxyphenyl)-3-methanol Butan-1-one (compound 3) 61 mg, yield 58%.
[0044] Product characterization: pale yellow liquid; 1 H NMR (500MHz, CDCl 3 )δ7.92–7.87(m,2H),7.48–7.42(m,2H),3.91(s,1H),3.12(s,2H),1.35(s,6H). 13 C NMR (125MHz, CDCl 3 )δ200.3, 140.1, 135.6, 129.5, 129.0, 69.8, 48.7, 29.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com