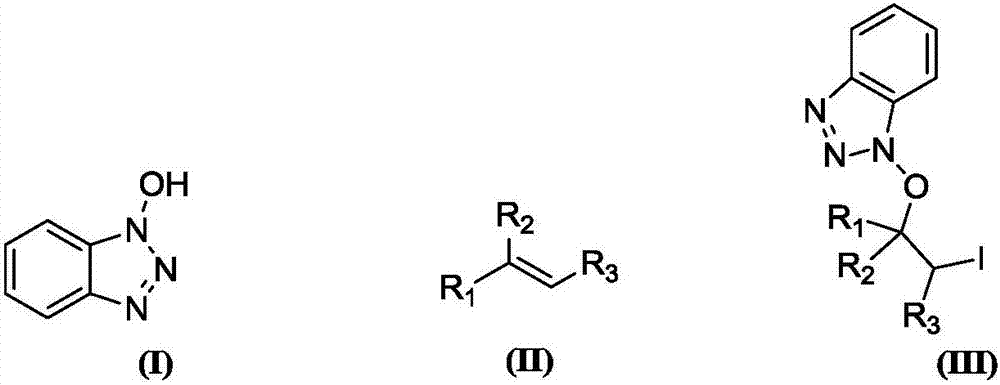
Synthesis method of beta-iodo-N-alkoxy benzotriazole compounds
A technology of hydroxybenzotriazole and synthesis method, applied in the direction of organic chemistry, etc., to achieve the effects of simple reaction operation, mild reaction conditions, and avoiding metal residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
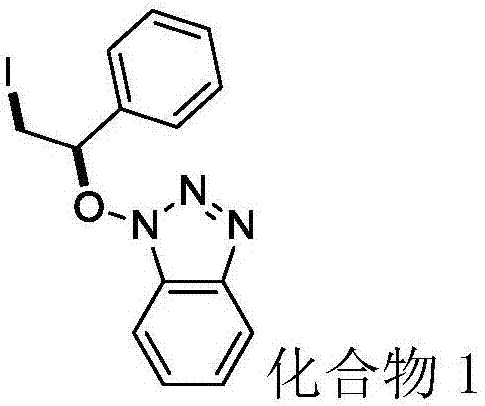
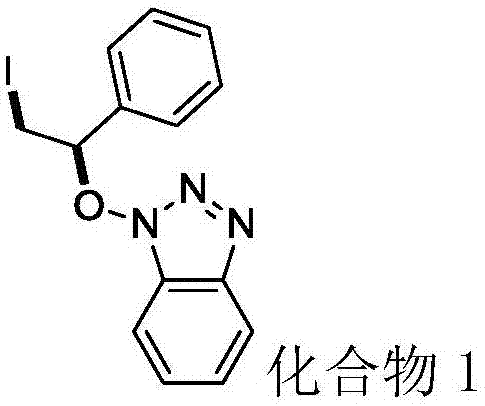
[0030] N-hydroxybenzotriazole (40.5mg, 0.3mmol), styrene (93.6mg, 0.9mmol), elemental iodine (38.1mg, 0.15mmol) and tert-butyl hydroperoxide (77.2mg, 0.6mmol, 70% aqueous solution) into the flask, add 2ml of solvent 1,2-dichloroethane, and react at 50°C for 4 hours. After the reaction was detected by TLC, compound 1 (83.3 mg) was isolated by column chromatography (eluent: petroleum ether / ethyl acetate volume ratio 6:1), with a yield of 76%.
[0031] Product characterization: colorless liquid; 1 H NMR (500MHz, CDCl 3 )δ7.93 (d, J = 8.3Hz, 1H), 7.39–7.32 (m, 6H), 7.29 (ddd, J = 8.0, 7.5, 3.0Hz, 2H), 5.69 (t, J = 7.0Hz, 1H ),3.92(dd,J=10.8,7.1Hz,1H),3.71(dd,J=10.8,7.0Hz,1H). 13 C NMR (125MHz, CDCl 3 ) δ143.12, 135.4, 130.1, 128.8, 127.90 (d, J=5.8Hz), 127.5, 124.4, 120.0, 108.8, 91.8, 2.9. HRMS (ESI) calcd for C 14 h 16 IN 4 O (M+NH 4 + )383.0369, found 383.0366.
Embodiment 2
[0033]
[0034] N-hydroxybenzotriazole (40.5mg, 0.3mmol), styrene (156mg, 1.5mmol), elemental iodine (38.1mg, 0.15mmol) and tert-butyl hydroperoxide (77.2mg, 0.6mmol, 70 % aqueous solution) into the flask, add 2ml of solvent 1,2-dichloroethane, and react at 50°C for 4 hours. After the reaction was detected by TLC, compound 1 (83.3 mg) was isolated by column chromatography (eluent: petroleum ether / ethyl acetate volume ratio 6:1), with a yield of 76%.
[0035] Product characterization: colorless liquid; 1 H NMR (500MHz, CDCl 3)δ7.93 (d, J = 8.3Hz, 1H), 7.39–7.32 (m, 6H), 7.29 (ddd, J = 8.0, 7.5, 3.0Hz, 2H), 5.69 (t, J = 7.0Hz, 1H ),3.92(dd,J=10.8,7.1Hz,1H),3.71(dd,J=10.8,7.0Hz,1H). 13 C NMR (125MHz, CDCl 3 ) δ143.12, 135.4, 130.1, 128.8, 127.90 (d, J=5.8Hz), 127.5, 124.4, 120.0, 108.8, 91.8, 2.9. HRMS (ESI) calcd for C 14 h 16 IN 4 O (M+NH 4 + )383.0369, found 383.0366.
Embodiment 3
[0037]
[0038] N-hydroxybenzotriazole (40.5mg, 0.3mmol), styrene (41.2mg, 0.9mmol), elemental iodine (76.2mg, 0.3mmol) and tert-butyl hydroperoxide (77.2mg, 0.6mmol, 70% aqueous solution) into the flask, add 2ml of solvent 1,2-dichloroethane, and react at 50°C for 4 hours. After the reaction was detected by TLC, compound 1 (83.3 mg) was isolated by column chromatography (eluent: petroleum ether / ethyl acetate volume ratio 6:1), with a yield of 76%.
[0039] Product characterization: colorless liquid; 1 H NMR (500MHz, CDCl 3 )δ7.93 (d, J = 8.3Hz, 1H), 7.39–7.32 (m, 6H), 7.29 (ddd, J = 8.0, 7.5, 3.0Hz, 2H), 5.69 (t, J = 7.0Hz, 1H ),3.92(dd,J=10.8,7.1Hz,1H),3.71(dd,J=10.8,7.0Hz,1H). 13 C NMR (125MHz, CDCl 3 ) δ143.12, 135.4, 130.1, 128.8, 127.90 (d, J=5.8Hz), 127.5, 124.4, 120.0, 108.8, 91.8, 2.9. HRMS (ESI) calcd for C 14 h 16 IN 4 O (M+NH 4 + )383.0369, found 383.0366.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com