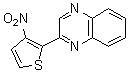
Palladium-catalyzed ortho-orientation nitrification method of aza calixarene compounds
A technology for azaaromatics and compounds, which is applied in the field of ortho-directed nitration of azaaromatics, can solve problems such as inconvenient operation, and achieve good adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
[0044] Combine 103 mg (0.5 mmol) of 2-phenylquinoxaline, 11 mg (0.05 mmol) of palladium diacetate, 154 mg (1.0 mmol) of silver nitrite, 270 mg (1.0 mmol) of potassium persulfate and 1,2-bis Chloroethane (5 ml) was sequentially added to a 10 ml sealed pressure vessel. The mixture was heated and reacted in an oil bath at 130°C for 48 hours. After the reaction is detected by TLC, the reaction solution is diluted with dichloromethane and filtered to obtain a clear liquid, which is separated by column chromatography (eluent ratio: petroleum ether to ethyl acetate volume ratio 4:1) to obtain a yellow solid 2-( 2-Nitrophenyl)quinoxaline 107 mg (86% yield).
[0045] Characterization data: mp 114-115 ℃; IR (KBr): n = 1531 (NO 2 ) cm -1 ; 1 H NMR (CDCl 3 , 500 MHz): δ 8.97 (s, 1H), 8.18-8.10 (m, 3H), 7.83-7.68 (m, 5H); 13 C NMR (CDCl 3 , 125 MHz): δ 151.1, 148.8, 144.3, 141.8, 141.6, 133.3, 133.0, 131.9, 130.6, 130.4, 130.3, 129.6, 129.3, 125.0; MS (EI, 70eV): m / z (%) = 251 (83)...
Embodiment 2
[0047]
[0048] Combine 103 mg (0.5 mmol) of 2-phenylquinoxaline, 11 mg (0.05 mmol) of palladium diacetate, 154 mg (1.0 mmol) of silver nitrite, 270 mg (1.0 mmol) of potassium persulfate and dichloromethane (5 ml) in order to add 10 ml sealed pressure vessel. The mixture was heated and reacted in an oil bath at 130°C for 48 hours. After the reaction is detected by TLC, the reaction solution is diluted with dichloromethane and filtered to obtain a clear liquid, which is separated by column chromatography (eluent ratio: petroleum ether to ethyl acetate volume ratio 4:1) to obtain a yellow solid 2-( 2-Nitrophenyl)quinoxaline 102 mg (81% yield).
Embodiment 3
[0050]
[0051] Combine 103 mg (0.5 mmol) of 2-phenylquinoxaline, 35 mg (0.05 mmol) of bis(triphenylphosphine) palladium dichloride, 154 mg (1.0 mmol) of silver nitrite, and 270 mg (1.0 mmol) of potassium persulfate. mmol) and 1,2-dichloroethane (5 ml) were sequentially added to a 10 ml sealed pressure vessel. The mixture was heated and reacted in an oil bath at 130°C for 48 hours. After the reaction is detected by TLC, the reaction solution is diluted with dichloromethane and filtered to obtain a clear liquid, which is separated by column chromatography (eluent ratio: petroleum ether to ethyl acetate volume ratio 4:1) to obtain a yellow solid 2-( 2-Nitrophenyl)quinoxaline 91 mg (72% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com