Nitration method of aromatic hydrocarbon compound
A technology for aromatic hydrocarbon compounds and nitric acid, applied in the field of nitrification of aromatic hydrocarbon compounds, can solve the problems of waste of resources, long reaction time, instability, etc., and achieve the effects of operator safety, reduction of workshop material storage, and less waste acid wastewater.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
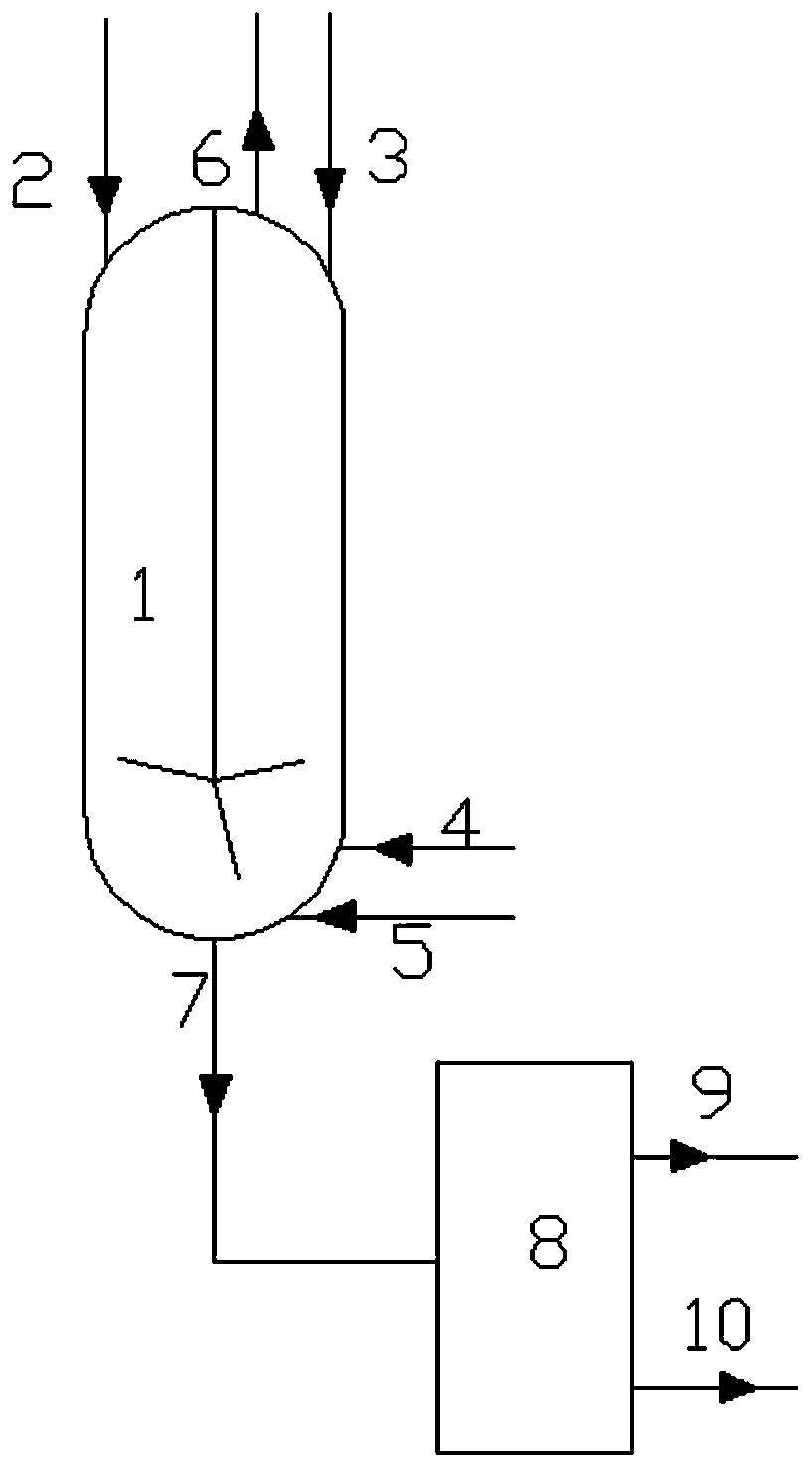
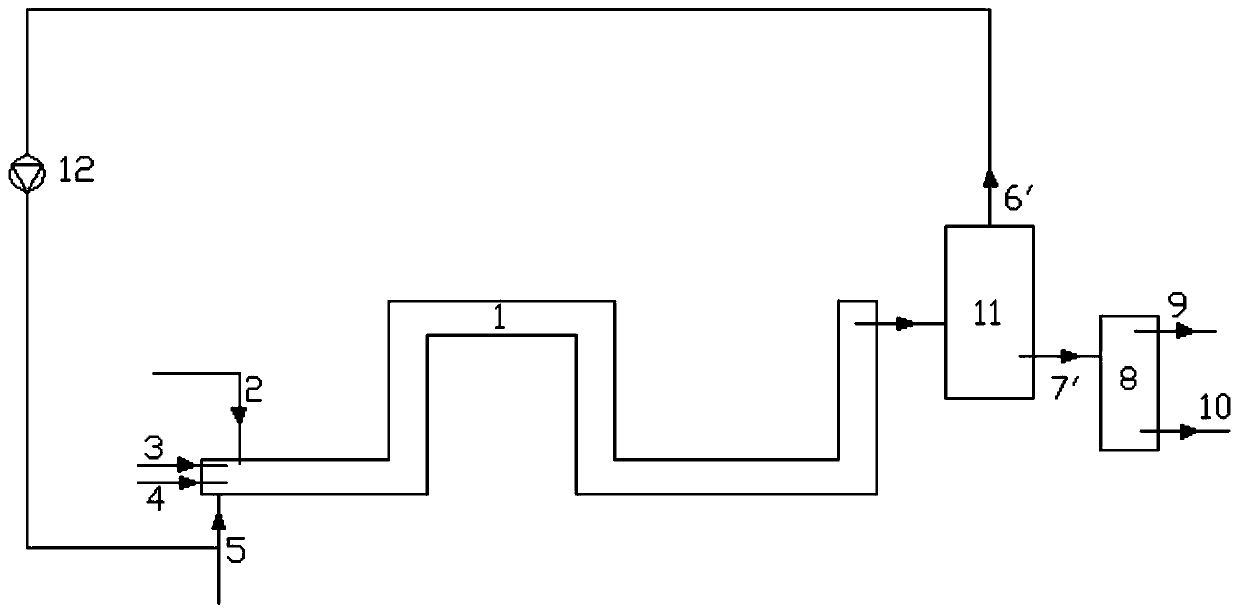
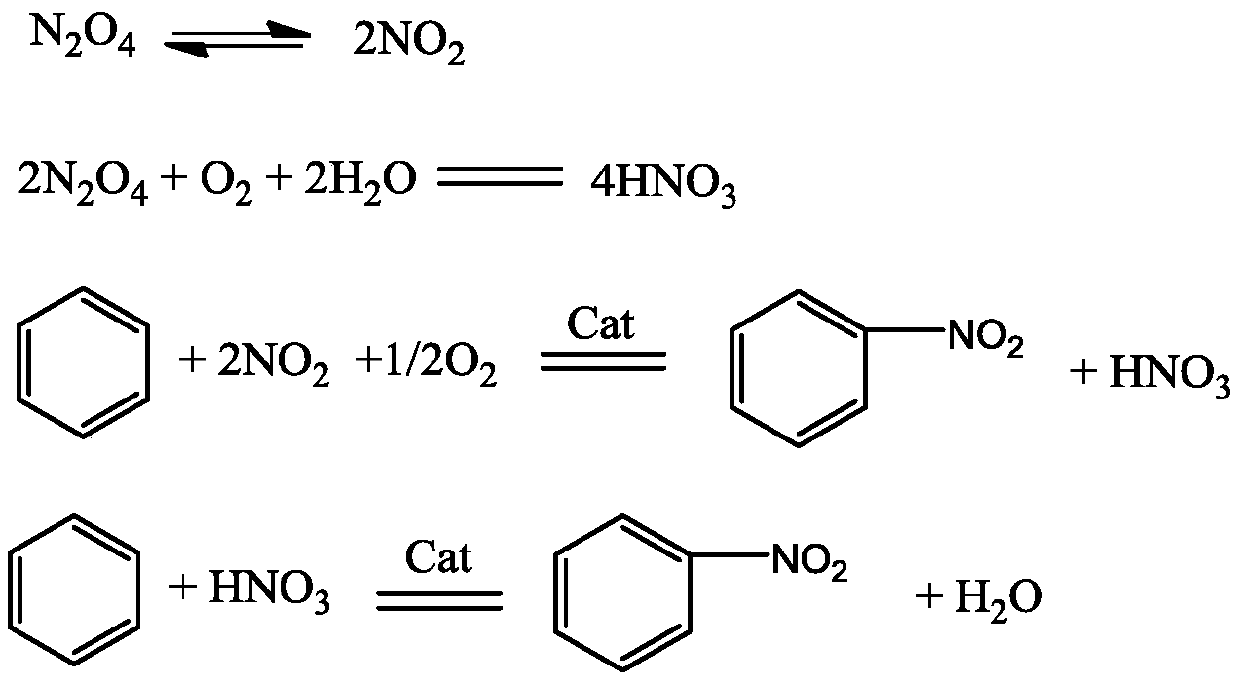
[0038] like figure 1 Shown, a kind of nitration method of aromatic compound comprises the following steps:
[0039] 1) In the reactor 1 having a temperature-measuring reflux device and band stirring, at first drop into 15.6 grams of solid acid catalyst, then add 312.4 grams of aromatic compound in reactor 1 through the aromatic compound feeding pipe 3, and pass through the liquid dioxygen tetraoxide Nitrogen feed pipe 4 drips 202.4 grams of liquid dinitrogen tetroxide in reactor 1, simultaneously feeds 32 grams of oxygen into reactor 1 by bubbling oxygen-containing flow inlet 5, and controls reaction pressure at 6MPa, temperature at 50°C;
[0040] 2) while step 1) is carried out, dropwise the sulfuric acid of 59.2 grams of 95% in reactor 1 by sulfuric acid feed pipe 2;
[0041] 3) The material obtained from the liquid outlet 7 enters the separator 8, and the aqueous phase material 9 and the organic phase material 10 are obtained through liquid separation treatment, and the s...
Embodiment 2
[0046] The reaction steps are the same as in Example 1, except that in step 2), 8.7 grams of nitric acid with a concentration of 65% and 59.2 grams of 95% sulfuric acid are configured into a mixed acid and then added to the reactor 1 together.
[0047] The average residence time of the reactants was 1.4 hours, and the yield of nitrobenzene was 95.9% based on benzene. After the catalyst was used continuously for 20 times, the yield of nitrobenzene was 94.34% based on benzene; after the catalyst was used continuously for 40 times, the catalyst still maintained good catalytic activity, and the yield of nitrobenzene was 92.8% based on benzene.
Embodiment 3
[0049] The reaction steps are the same as in Example 1, except that 5.6 grams of water and liquid dinitrogen tetroxide are dropped into the reactor 1 simultaneously in the step 1), and the addition of process conditions and other substances is also the same as in Example 1.
[0050] The average residence time of the reactants was 1.9 hours, and the yield of nitrobenzene was 95.72% based on benzene. After the catalyst was used continuously for 20 times, the yield of nitrobenzene was 93.72% based on benzene; after the catalyst was used continuously for 40 times, the catalyst still maintained good catalytic activity, and the yield of nitrobenzene was 90.03% based on benzene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com