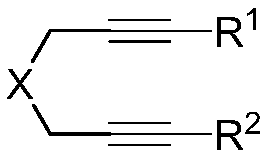
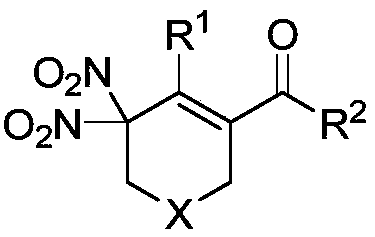
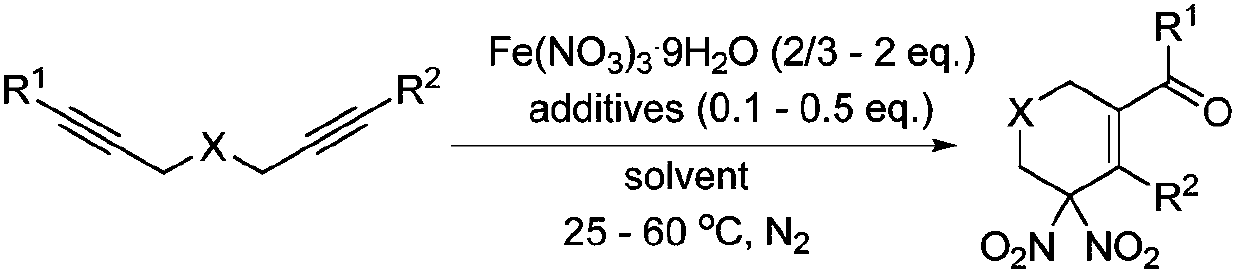
Method of using nitrate hydrate to prepare bisphosphonate nitro compound
A technology for dinitro compounds, which is applied in the preparation of nitro compounds, the formation/introduction of nitro/nitroso groups, and organic chemistry, etc., can solve the problems of waste in industrial production, a large number of strong bases, and the environment is not friendly enough to achieve The effect of good application prospect, less additive dosage and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] In a dry Schlenk tube, add 92 mg of the above diacetylene, 81 mg of Fe(NO 3 ) 3 9H 2 O and 9 mg of pyridine N-oxide. The Schlenk tube was evacuated first, then filled with nitrogen, and repeated three times. Then, 0.5 mL of nitromethane was added to the Schlenk tube, and the resulting reaction solution was stirred at room temperature for 4 hours. After the reaction was completed, it was filtered with diatomaceous earth, concentrated, and passed through a silica gel column (the volume ratio of petroleum ether to ethyl acetate was 20:1), and 97 mg of the product was obtained with a yield of 85%.
[0033] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0034] 1 H NMR (400MHz, CDCl 3 )δ7.80(d, J=8.8Hz, 2H), 7.70(d, J=8.4Hz, 2H), 7.40(d, J=8.0Hz, 2H), 7.04(d, J=8.8Hz, 2H) ,6.82(d,J=8.8Hz,2H),6.59(d,J=8.8Hz,2H),4.42(s,2H),4.12(s,2H),3.83(s,3H),3.64(s, 3H), 2.49(s, 3H).
[0035] 13 C NMR (100MHz, CDCl 3 )δ...
Embodiment 2
[0037]
[0038] In a dry Schlenk tube, add 80 mg of the above diacetylene, 81 mg of Fe(NO 3 ) 3 9H 2O and 4mg p-benzoquinone. The Schlenk tube was evacuated first, then filled with nitrogen, and repeated three times. Then, 0.5 mL of acetonitrile was added to the Schlenk tube, and the resulting reaction solution was stirred at room temperature for 26 hours. After the reaction was completed, the mixture was filtered with diatomaceous earth, concentrated, and passed through a silica gel column (the volume ratio of petroleum ether to ethyl acetate was 20:1) to obtain 60 mg of the product with a yield of 59%.
[0039] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0040] 1 H NMR (400MHz, CDCl 3 )δ7.77(d, J=8.4Hz, 2H), 7.72(d, J=8.4Hz, 2H), 7.51(t, J=7.6Hz, 1H), 7.44(d, J=8.0Hz, 2H) ,7.37(t,J=7.6Hz,2H),7.09-7.05(m,5H),4.46(s,2H),4.18(s,2H),2.50(s,3H).
[0041] 13 C NMR (100MHz, CDCl 3 )δ193.76, 145.50, 145.09, 134.63, 13...
Embodiment 3
[0043]
[0044] In a dry Schlenk tube, add 85 mg of the above diyne and 162 mg of Fe(NO 3 ) 3 9H 2 O. The Schlenk tube was evacuated first, then filled with nitrogen, and repeated three times. Then, 0.5 mL of acetonitrile solution containing 2.5 μl of concentrated nitric acid was added to the Schlenk tube, and the resulting reaction solution was stirred at room temperature for 8 hours. After the reaction was completed, it was filtered with diatomaceous earth, concentrated, and passed through a silica gel column (the volume ratio of petroleum ether to ethyl acetate was 20:1), to obtain 44 mg of the product with a yield of 42%.
[0045] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0046] 1 H NMR (400MHz, CDCl 3 )δ7.71-7.68 (m, 4H), 7.43 (d, J = 8.4Hz, 2H), 7.17 (d, J = 8.4Hz, 2H), 6.98-6.85 (dd, J 1 =8.4Hz,J 2 =43.2Hz,4H),4.43(s,2H),4.13(s,2H),2.49(s,3H),2.35(s,3H),2.13(s,3H).
[0047] 13 C NMR (100MHz, CDCl 3 )δ1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com