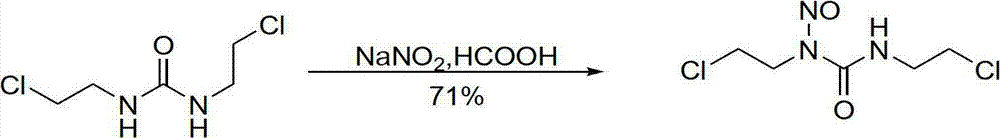Method for synthesizing nitrosoureas antineoplastic drugs through stannic chloride-sodium nitrite system
An anti-tumor drug and sodium nitrite technology, which is applied in the field of synthesizing nitrosourea anti-tumor drugs with the tin tetrachloride-sodium nitrite system, can solve the problems of low yield and cumbersome post-processing, and achieve cost reduction and responsiveness. The effect of fast speed and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] a. SnCl at room temperature 4 ·5H 2 O (3.5g, 10mmol) solid, 1,3-bis(2-chloroethyl)urea (1.9g, 10mmol) and sodium nitrite (0.7g, 10mmol) were added to 30ml of dichloromethane, stirred for 2 hours .
[0048] b, filter to remove the insoluble matter, and the filtrate is washed with saturated NaHCO 3 Solution washing, anhydrous Na 2 SO 4dry.
[0049] c. The filtrate was filtered to remove the desiccant, dichloromethane was evaporated with a rotary evaporator, and the obtained solid was recrystallized with isopropanol. Carmustine (1.9 g, 9.1 mmol) was obtained with a yield of 91%.
Embodiment 2
[0051] a. SnCl at room temperature 4 ·5H 2 O (15.8g, 45mmol) solid, 1,3-bis(2-chloroethyl) urea (5.6g, 30mmol) and sodium nitrite (3.1g, 45mmol) were added to 80ml of carbon tetrachloride, stirred for 3.5 Hour.
[0052] b, filter to remove the insoluble matter, and the filtrate is washed with saturated KHCO 3 Solution washing, anhydrous Na 2 SO 4 dry.
[0053] c. The filtrate was filtered to remove the desiccant, and the carbon tetrachloride was evaporated with a rotary evaporator, and the obtained solid was recrystallized with a mixed solvent of water-acetone (10ml: 40ml). Carmustine (5.8 g, 27.3 mmol) was obtained with a yield of 91%.
Embodiment 3
[0055] a. SnCl at room temperature 4 ·5H 2 O (35g, 100mmol) solid, 1,3-bis(2-chloroethyl)urea (9.3g, 50mmol) and sodium nitrite (7g, 100mmol) were added to 150ml 1,2-dichloroethane, stirred React for 5 hours.
[0056] b. Filter to remove the insoluble matter, and the filtrate is washed with saturated KH 2 PO 4 Solution washing, anhydrous Na 2 SO 4 dry.
[0057] c. Remove the desiccant from the filtrate, evaporate 1,2-dichloroethane with a rotary evaporator, and recrystallize the obtained solid with a mixed solvent of water-ethanol (5ml︰45ml). Carmustine (9.7 g, 45.5 mmol) was obtained with a yield of 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com