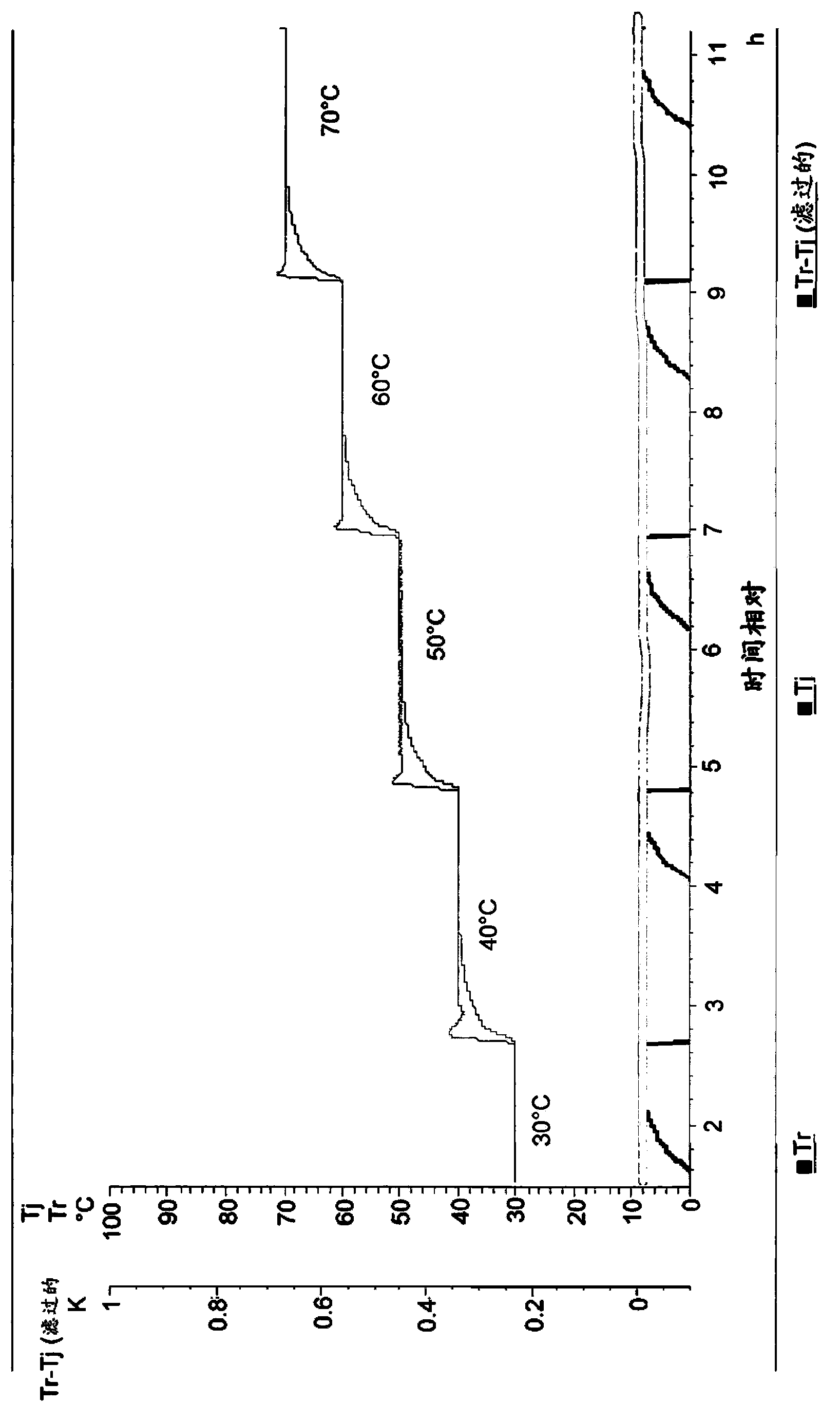
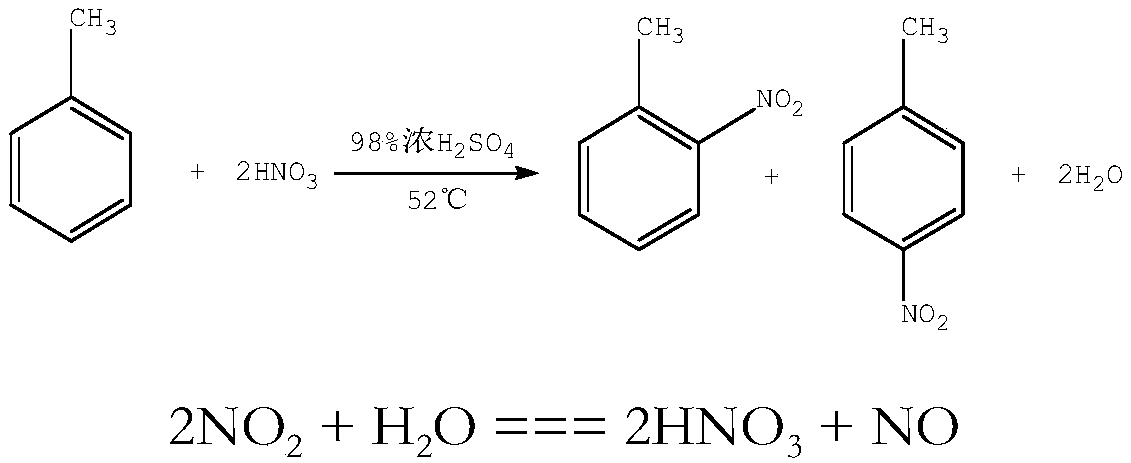
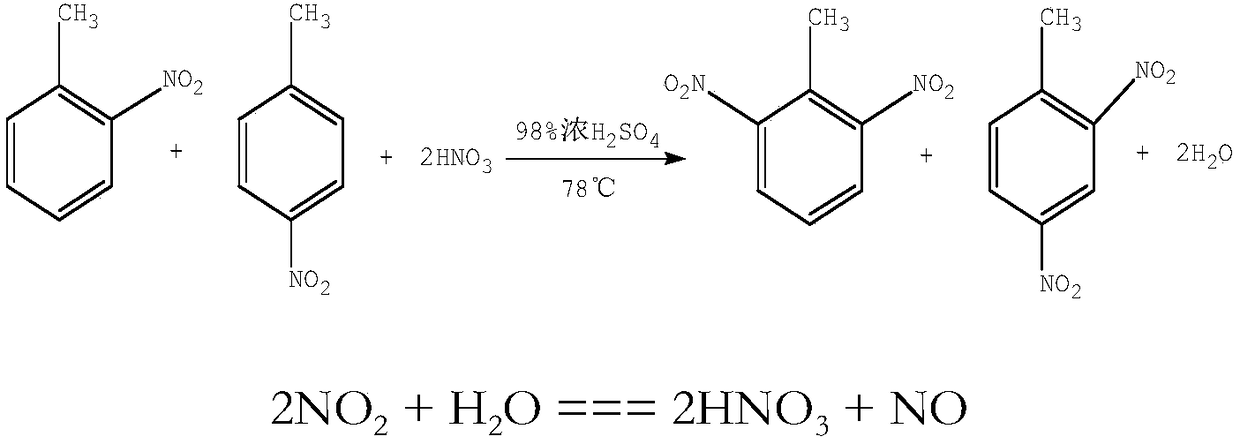
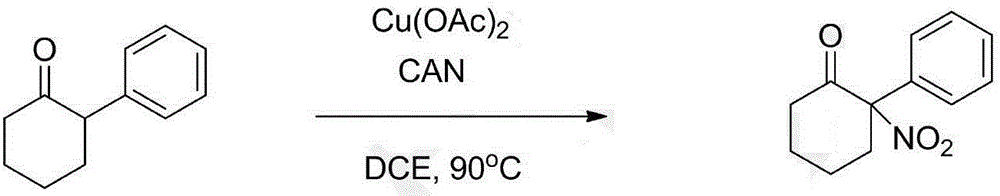
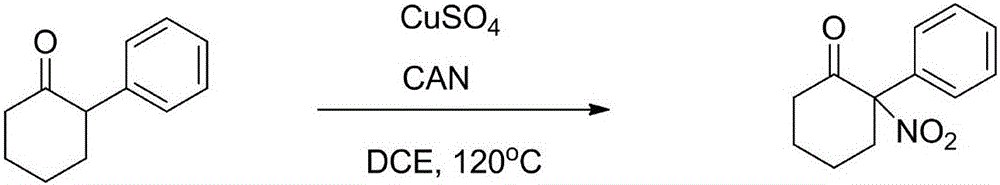
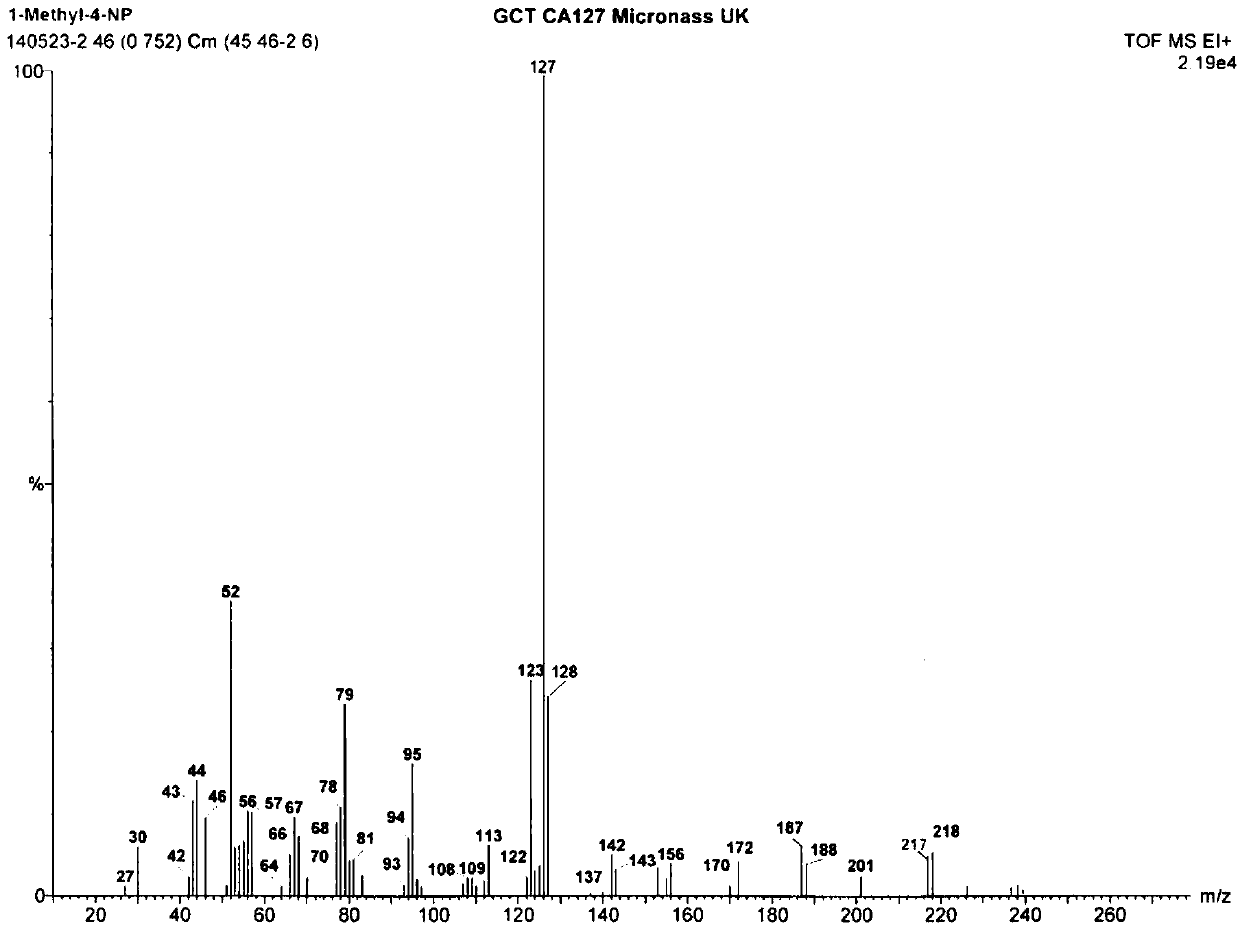
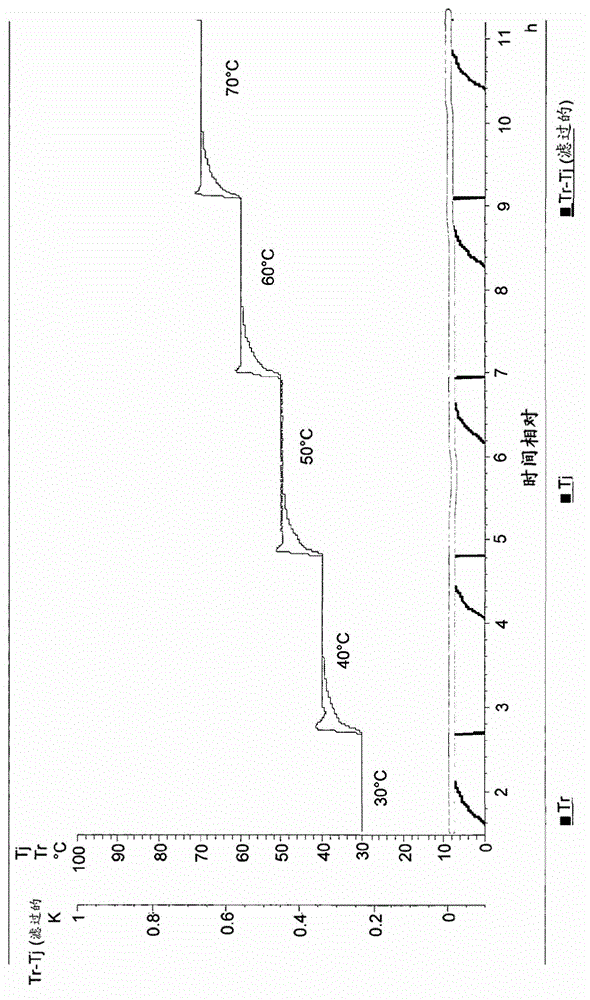
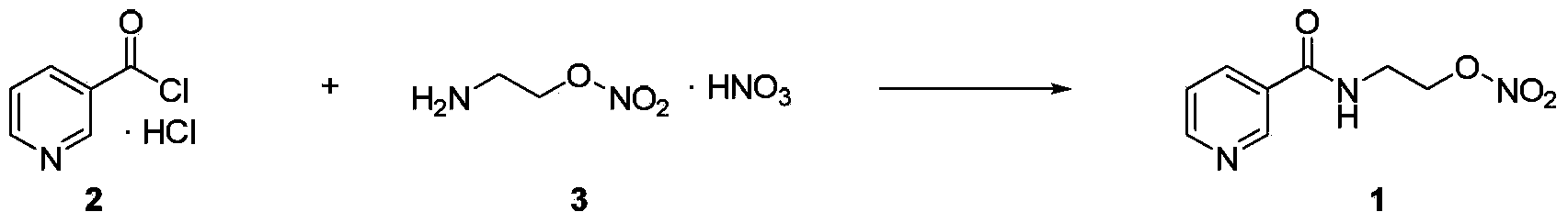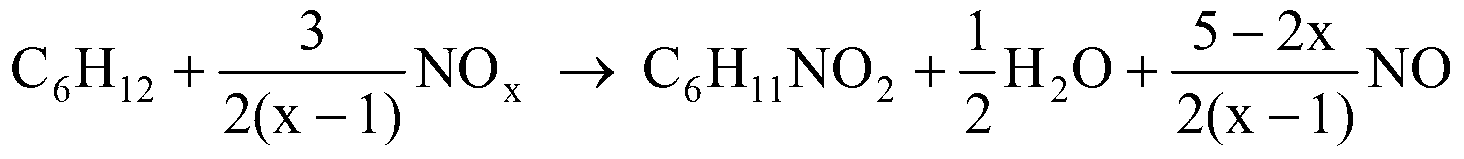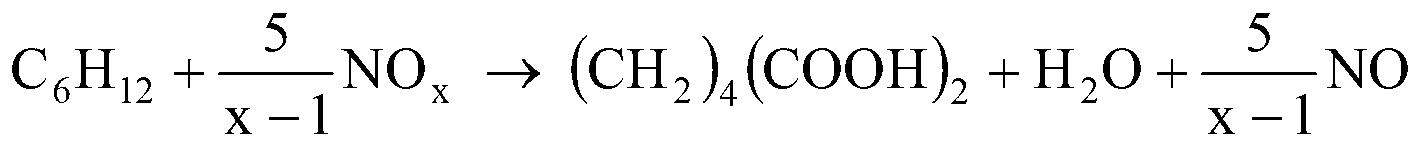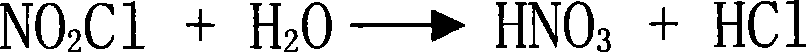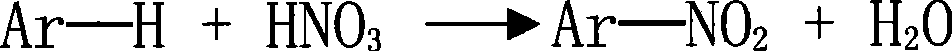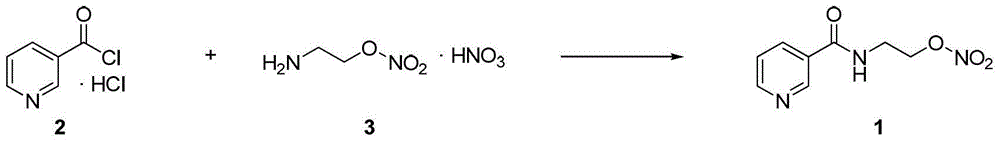Patents
Literature
79 results about "Nitrate agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
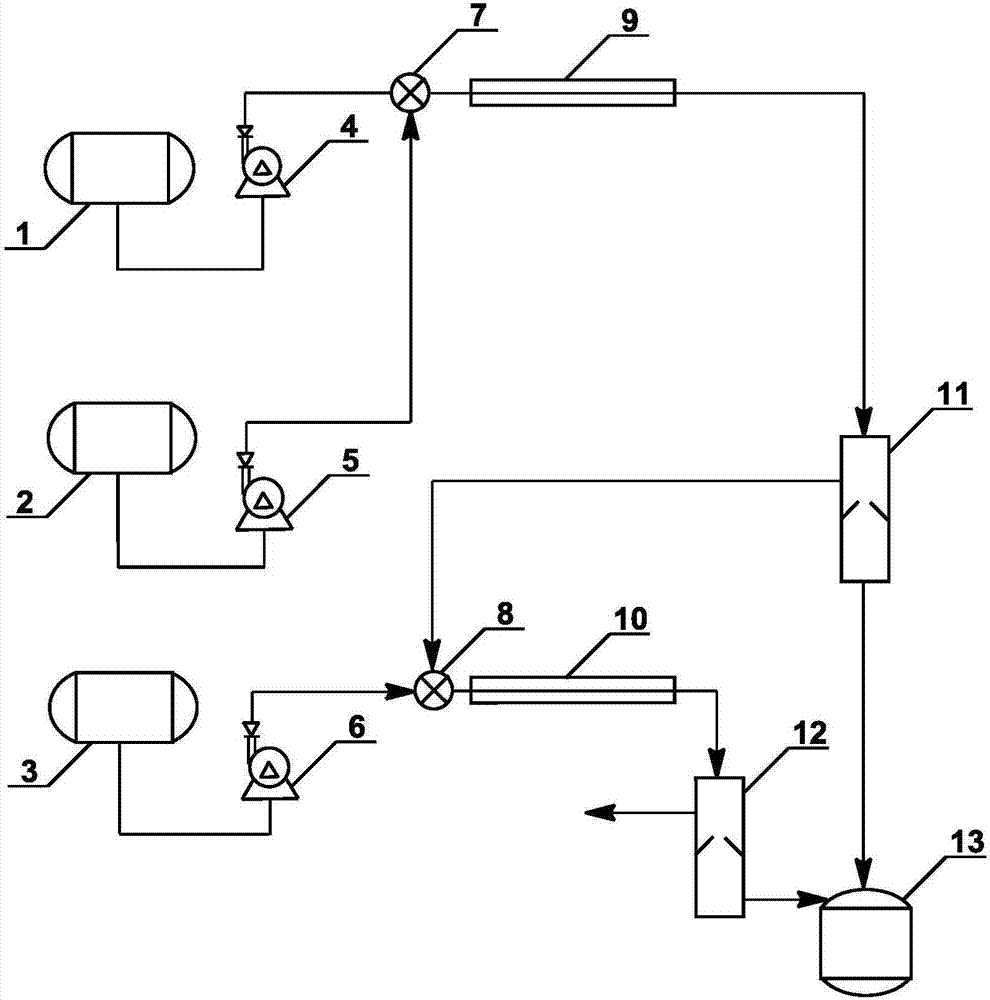
Method for synthesizing isooctyl nitrate and microchannel reactor
ActiveCN101462962AAvoid production accidentsLow costNitric acid ester preparationSynthesis methodsSolvent
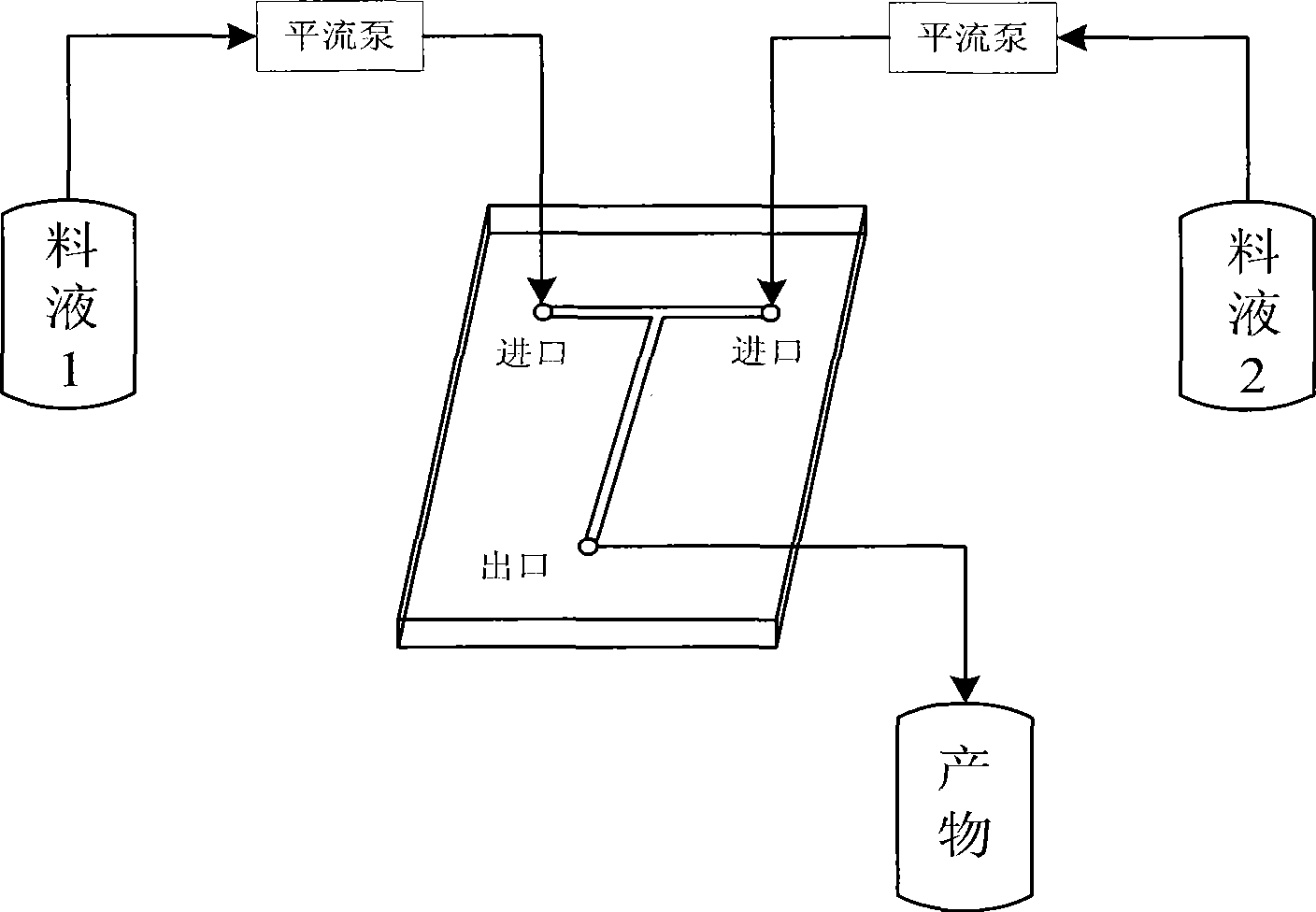
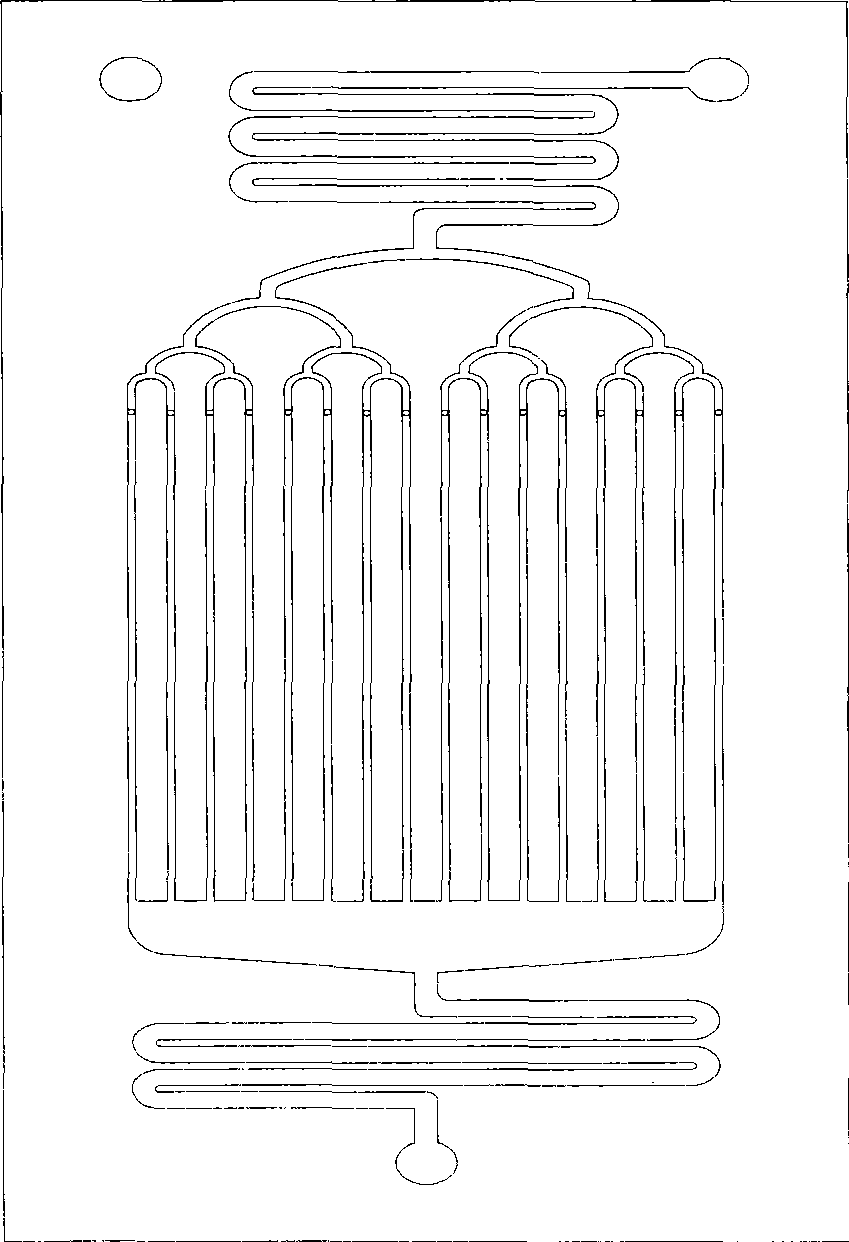
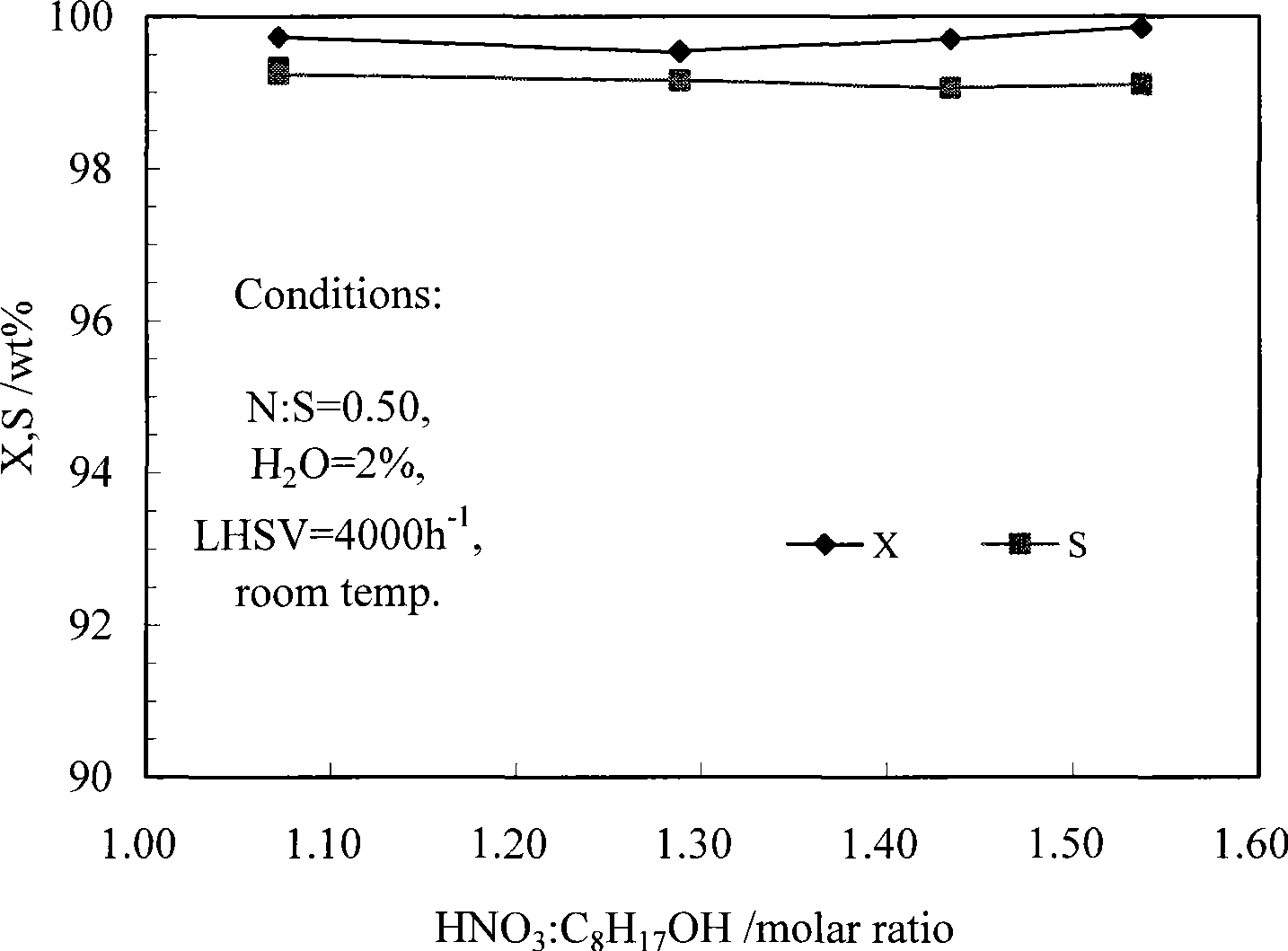
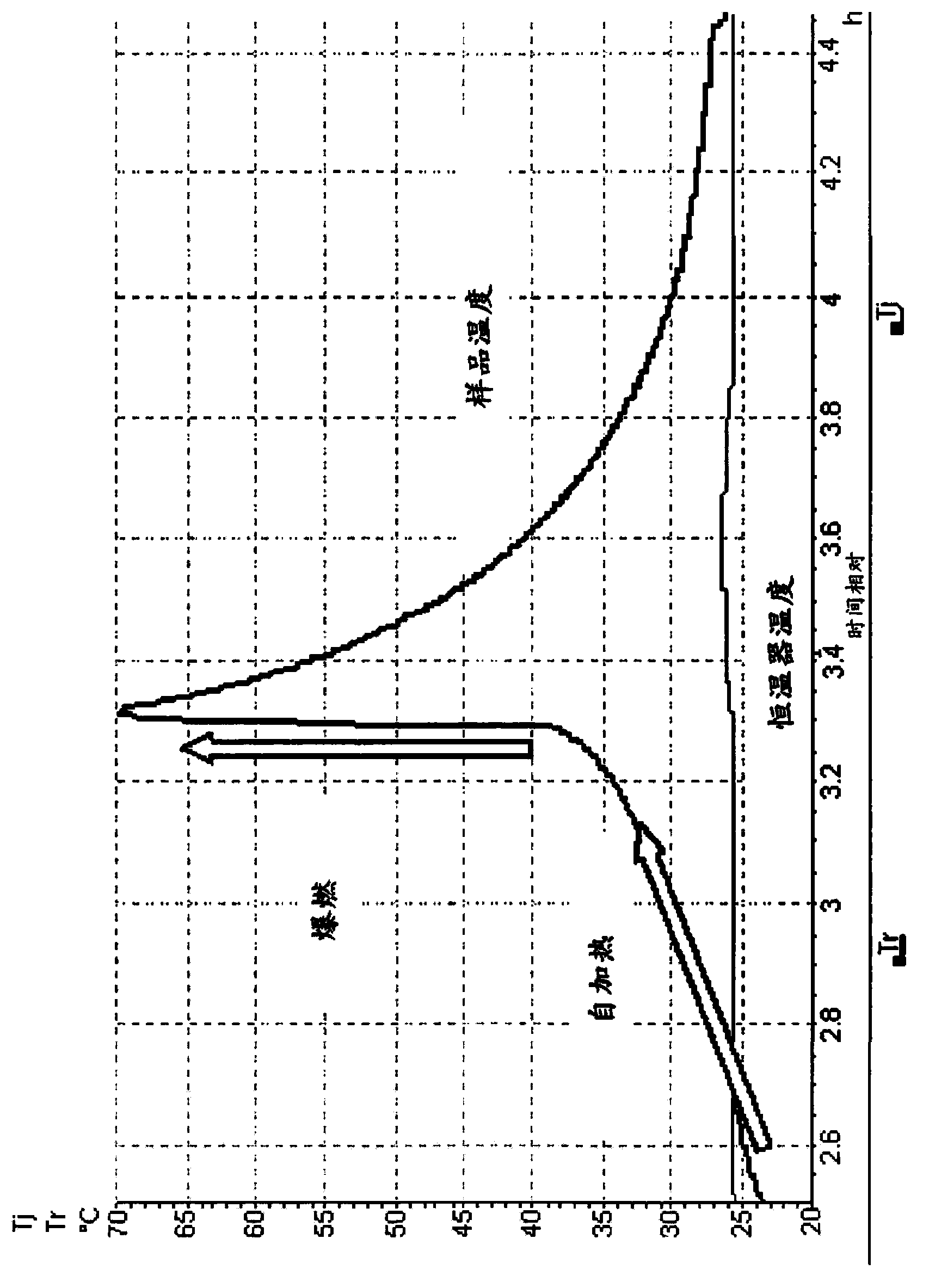
The invention relates to the synthesis of isooctyl nitrate; in particular to a synthesis method of isooctyl nitrate and a microchannel reactor. Isooctyl alcohol is taken as raw material and an acid mixture of nitric acid and sulphuric acid is taken as nitrating agent, and the raw material and the nitrating agent are pumped into the microchannel reactor at normal temperature and pressure to carry out direct esterification. The process needs no catalyst or solvent, makes full use of the efficient heat and mass transfer capability of the microchannel as well as the characteristic of direct amplification and adopts two microreactors, of which one is a single-channel microreactor and the other is a multichannel microreactor; liquid hourly space velocity in the microreactor is 3000-10000h<-1>, the once-through conversion ratio of isooctyl alcohol is higher than 99% and the yield coefficient of isooctyl nitrate is higher than 98%. The process has high operation safety and high selectivity and can realize approximate isothermal operation in the reaction. The reactor is small in volume, is easy to integrate and amplify and space-time productive rate is high, thus being significant for strengthening and guaranteeing the safe production of isooctyl nitrate.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Use of bismuth nitrate and iron nitrate as nitrification agent in aromatic compound nitrification
InactiveCN1854114AChemically stableLess corrosiveNitro/nitroso group formation/introductionNitro compound preparationNitrateNitrate agent
Owner:北京清华紫光英力化工技术有限责任公司
Nitration of activated aromatics in microreactors
InactiveCN101400628AHigh yieldHigh mass fluxChemical/physical/physico-chemical microreactorsNitro/nitroso group formation/introductionMicroreactorNitration
The invention relates to the nitration of aromatic or heteroaromatic compounds, wherein an activated aromatic or heteroaromatic compound and a nitrating agent, in the presence, if desired, of a solvent, are mixed intensely in a microreactor, and wherein the proportion of the nitrating agent to the activated aromatic or heteroaromatic compound, the concentration of nitrating agent in the reaction mixture, and the temperature are selected at levels such that the nitration begins autocatalytically, and wherein the nitration product is obtained after leaving the microreactor and, if desired, after an after-reaction time outside the microreactor.
Owner:LONZA LTD
Process for the manufacture of nicorandil
Disclosed is a process for the synthesis of Nicorandil (1), 2-(nicotinamide)ethyl nitrate, starting from N-(2-hydroxyethyl)nicotinamide (15), using nitration with nitric acid in the presence of acetic anhydride Said synthesis method is particularly advantageous because it solves the safety problems involved in the use of nitric acid as nitrating agent, and allows a product with excellent yields and quality to be isolated.
Owner:PROCOS
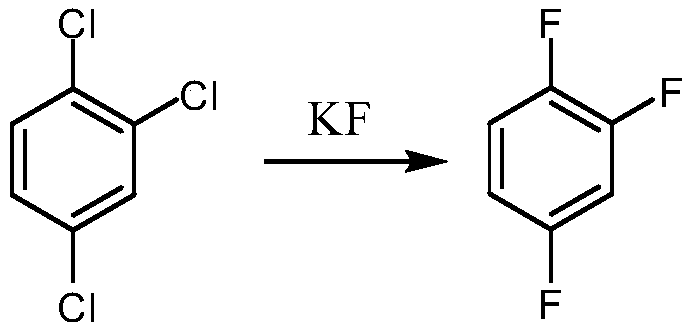
Synthetic method for 1,2,4-trifluorobenzene
ActiveCN110498730ALow costEasy to realize industrializationOrganic compound preparationSulfuric acid amide preparationNitrosylsulfuric acidPotassium fluoride
The invention provides a synthetic method for 1,2,4-trifluorobenzene, belongs to the field of pesticide, medicine, and liquid crystal material intermediate preparation, and solves the problem of harshreaction conditions of a current method for synthesizing 1,2,4-trifluorobenzene. The synthetic method for the 1,2,4-trifluorobenzene is characterized by comprising the following steps: performing nitration by using 2,4-dichlorofluorobenzene as a raw material and nitric acid as a nitrating agent to form 2,4-dichloro-5-fluoronitrobenzene in the presence of sulfuric acid; dissolving the 2,4-dichloro-5-fluoronitrobenzene into an organic solvent, adding potassium fluoride and a first catalyst, and performing fluorination under the catalysis of the first catalyst to obtain 2,4,5-trifluoronitrobenzene; dissolving the 2,4,5-trifluoronitrobenzene into a solvent, and performing hydrogenation reduction with hydrogen under the catalysis of a second catalyst to obtain 2,4,5-trifluoroaniline; and performing a reaction on the 2,4,5-trifluoroaniline and sulfuric acid, after a salt is formed, performing a diazotization reaction on the salt and nitroso-sulfuric acid, performing a deamination reductionreaction with sodium hypophosphite under the catalysis of a copper salt, and finally performing steam distillation to obtain the 1,2,4-trifluorobenzene. The method provided by the invention has the advantages of mild reaction conditions and the like
Owner:ZHEJIANG LINJIANG CHEM
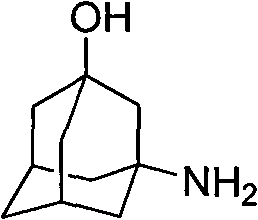
Method for preparing 3-amino-1-adamantane alcohol
InactiveCN101798270AReduce dosageReduce processing costsOrganic compound preparationAmino-hyroxy compound preparationWater bathsIce water
The invention relates to a method for preparing 3-amino-1-adamantane alcohol. The method comprises the following steps of: adding amantadine hydrochloride into a nitrating agent in batches, performing reaction for 1 to 2 hours in an ice-water bath and performing reaction for 1 to 30 hours at room temperature to obtain yellowish liquid; pouring the yellowish liquid into ice, continuously reacting for 0.5 to 2 hours with stirring to obtain blue-green liquid; and adding solid base into solution obtained by the step 2 with stirring, keeping temperature below 80 DEG C, regulating pH to be between 10 and 12, performing reaction for 30 minutes with stirring, leaching, extracting reaction liquid by using dichloromethane, drying the obtained product with anhydrous sodium sulfate, steaming off the dichloromethane and performing recrystallization by using ethyl acetate to obtain white solid. The preparation method has the advantages of readily available starting raw materials, simple reaction operation, short route, environmental friendliness, easy industrial production and good application prospect, and also reduces cost for the synthesis of Vildagliptin serving as a medicament for treatingdiabetes; and the yield of products reaches 75 percent.
Owner:DONGHUA UNIV
Linking dithizone resin and preparation method thereof
InactiveCN101381426AImprove stabilityImprove adsorption capacityOther chemical processesCross-linkNitration
The invention discloses chelating resin bonded with dithizone and a method for preparing the same. A functional group of the resin is a dithizone group the construction unit of which is as shown in the graph. The preparation method comprises the following steps: styrene is taken as a monomer, diethylene is taken as a cross linking agent, fluid wax and other substances are taken as a pore-forming agent, magnesium carbonate and other substance are taken as a dispersing agent, and benzoperoxide is taken as an initiator, nitrating agents with different composition proportions are added for nitration and reduction so as to obtain macroporous amino resin, and stannous chloride dihydrate, hydrochloric acid and ethanol are added for diazotization, and acetone solution of the dithizone and glacial acetic acid are added to introduce the functional group so as to obtain the chelating resin bonded with the dithizone. Compared with the loaded resin, the stability of the functional group of the resin is obviously improved, so that the resin has wide application prospect in fields such as the metal separation and pre-enriching in the trace analysis, the heavy metal pollution treatment and recovery in the environment.
Owner:NANJING UNIV
Production method for isooctyl nitrate
InactiveCN105418432AReduce generation costThe response is stable and easy to controlNitric acid ester preparationIsooctyl alcoholNitrate
The invention discloses a production method for isooctyl nitrate. The production method comprises steps: firstly, an acid mixture of sulfuric acid and nitric acid with a mol ratio of 1.5-2.5:1 is prepared as a nitrating agent; secondly, a mixed solution of isooctyl nitrate and isooctyl alcohol with a mass ratio of 0.8-2:1 is prepared; thirdly, the mixed solution of isooctyl nitrate and isooctyl alcohol is added in the acid mixture of sulfuric acid and nitric acid at a temperature of 10-30 DEG C, the reaction is carried out for 0.5-1h, the mixture is allowed to stand for separation, organic phase alkali washing is carried out, water washing is carried out, and an isooctyl nitrate product is prepared. The production method is advantaged by simple technology, low cost and easy industrialization.
Owner:JIANGXI SILINCO
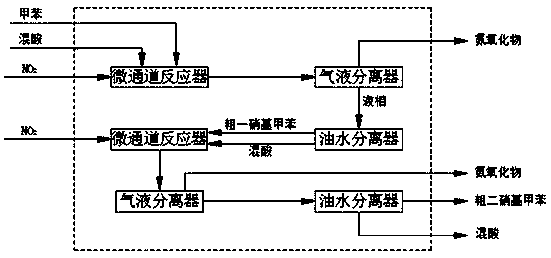
Method for synthesizing dinitrotoluene through micro-channel reactor
ActiveCN109467508AQuick responseIncrease reaction rateNitro compound preparationChemical industryGas phase
The invention belongs to the field of chemical industry process, and particularly discloses a method for synthesizing dinitrotoluene through a micro-channel reactor. The method comprises the followingsteps: feeding a measured mixed acid, NO2 gas and methylbenzene into the micro-channel reactor to mix and react fully in proportion; after the reaction is ended, performing gas-liquid separation first, further oxidizing oxynitride in a gas phase, and returning the oxynitride to the system to participate in the reaction again; standing a liquid phase for layering; respectively measuring an organicphase, an aqueous phase and the NO2, feeding the organic phase, the aqueous phase and the NO2 into a secondary micro-channel reactor for reacting again, performing gas-liquid separation on a secondary reaction product again, further oxidizing the oxynitride in the gas phase, and returning the oxynitride to the system to participate in the reaction again; standing the liquid phase for layering; returning the aqueous phase to participate in the reaction; performing cooling, alkaline washing, drying and purifying on the organic phase to obtain the dinitrotoluene product. According to the method,concentrated sulphuric acid is taken as a catalyst, and nitric acid and the NO2 are taken as nitrating agents; the mixed acid after the reaction can be recycled and used directly without concentrating, so that equipment investment is reduced, and the production energy consumption is obviously reduced.
Owner:SICHUAN GOLDEN ELEPHANT SINCERITY CHEM CO LTD
Method for catalyzing and synthesizing nitrobenzene by loading silicon dioxide with heteropoly acid ammonium
InactiveCN101538203AGood choiceEasy to separate and recyclePhysical/chemical process catalystsChemical recyclingReaction timingNitrate agent
The invention discloses a method for catalyzing and synthesizing nitrobenzene by loading silicon dioxide with heteropoly acid ammonium, which belongs to the technical field of organic synthesis. In the method, nitric acid with 65 percent of mass fraction concentration is used as a nitrating agent and a reaction is carried out under the conditions of normal pressure and temperature between 60 DEG C and 110 DEG C. The method uses the silicon dioxide loaded with the heteropoly acid ammonium as a catalyst and ensures that a mixed liquor of benzene and the nitric acid is stirred in a kettle type reactor to carry out a benzene nitration reaction, wherein the reaction time is between 1 hour and 7 hours. The method has 100 percent of nitration selectivity, eliminates the unsafe factor that the benzene nitration process generates explosive substances such as multiple nitrobenzene, oxide compounds, and the like and has the characteristics of high yield, reutilization of the catalyst and environmental protection.
Owner:LIAOCHENG UNIV
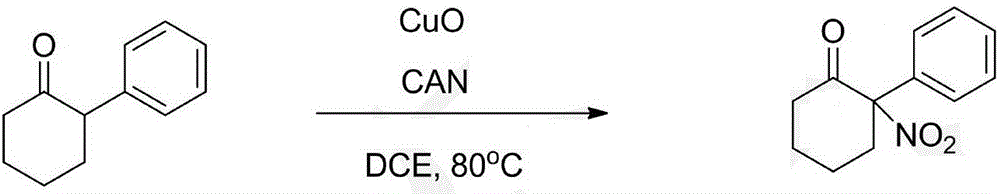
Synthesis method of alpha-nitrocycloalkanone
The invention discloses a synthesis method of alpha-nitrocycloalkanone. The structural formula of the alpha-nitrocycloalkanone is disclosed in the specification, wherein A is a five-ten-element ring, R is alkyl or aryl, and R' is hydrogen, alkyl, aryl or fused aryl. The method comprises the following step: under the catalytic action of a copper-base or iron-base catalyst, carrying out reaction on the compound disclosed in the specification and a nitrating agent to obtain the alpha-nitrocycloalkanone. By using the cheap copper-base or iron-base catalyst, the method can implement the synthesis of the alpha-nitrocycloalkanone economically and efficiently, and the reaction process is simple and safe and is easy to operate.
Owner:LANZHOU UNIVERSITY
Method for preparing N-alkyl-4-nitrophthalimide
ActiveCN109305933AReduce dosageReduce the discharge of three wastesOrganic chemistryOrganic synthesisReaction temperature
The invention belongs to the technical field of organic synthetic processes, and relates to a method for preparing N-alkyl-4-nitrophthalimide by adopting N-alkyl phthalimide as a raw material, nitricacid as a nitrating agent, and performing low-temperature nitration reaction by virtue of a continuous flow to prepare the series product of N-alkyl-4-nitrophthalimide. The method is short in reactiontime and short in production period, solves the problems produced by the accumulation of raw materials in an intermittent reaction kettle, is more stable in the reaction process, and can significantly improve the reaction efficiency. The mass transfer performance and heat conduction performance in a micro-channel reactor of different structures can be improved, the constant reaction temperature can be kept, the temperature runaway phenomenon can be avoided, the generation of byproducts can be reduced, and the safety of the reaction process can be improved. By adopting the high mass transfer effect in the micro-channel reactor, a liquid-liquid reaction solution is sufficiently mixed, in the reaction process, the consumption of concentrated sulfuric acid and nitric acid can be greatly reduced, and the generation of waste acid can be reduced.
Owner:ZHEJIANG WANFENG CHEM
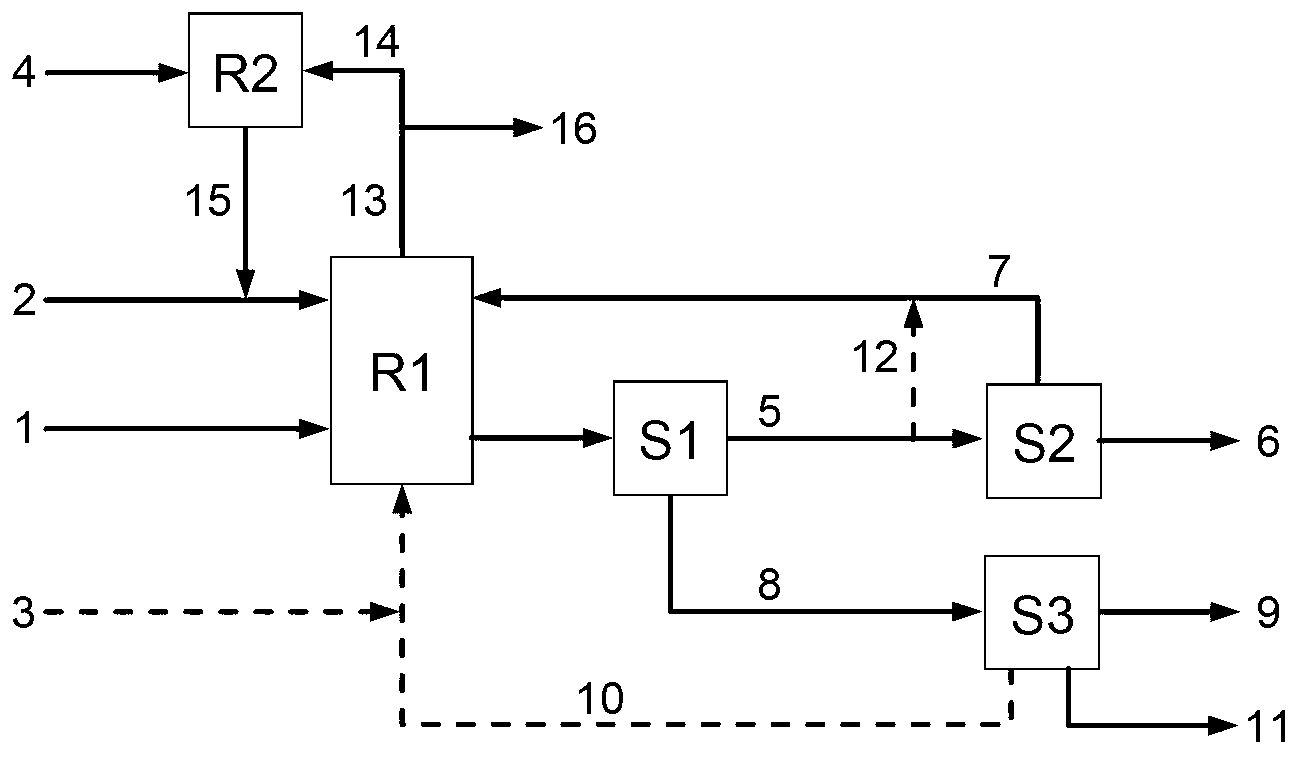
Method for co-producing adipic acid and nitrocyclohexane
ActiveCN103288626AHigh selectivityImprove conversion rateOrganic compound preparationCarboxylic compound preparationReaction temperatureAdipic acid
The present invention relates to a method for high selectivity co-production of adipic acid and nitrocyclohexane. According to the present invention, a nitrogen oxide NOx is adopted as an oxidant and a nitrating agent to carry out one step high selectivity conversion of cyclohexane into adipic acid and nitrocyclohexane, and adjustments of a reactant ratio, a reaction temperature, a reaction pressure, a type and an amount of a catalyst or an induction agent, and other conversion conditions are adopted to achieve regulation of a production ratio of the adipic acid and the nitrocyclohexane; and NO produced from the reaction can be recycled and then reacts with oxygen to be converted into NOx so as to be recycled.
Owner:XIANGTAN UNIV
Method for preparing o-nitrophenol through phenol nitration selectivity
InactiveCN102942487AIncreased nitrification conversionGood ortho selectivityPhysical/chemical process catalystsNitro compound preparationWater vaporHeteropoly acid
The invention discloses a method for preparing o-nitrophenol, which comprises the following steps: nitrating phenol in mild conditions with silica loaded heteropoly acid cesium as a catalyst and low-concentration nitric acid as a nitrating agent; and successively performing extraction, distillation, steam distillation and recrystallization to obtain the o-nitrophenol. The silica loaded heteropoly acid cesium is formed by loading 1% to 40% (wt%) of heteropoly acid cesium to silica. The method for preparing o-nitrophenol has the advantages of high phenol conversion rate, high o-phenol nitration selectivity, easiness in preparation of the catalyst, high catalytic activity, easy separation of the catalyst after the action, reusability and broad application prospects.
Owner:LIAOCHENG UNIV
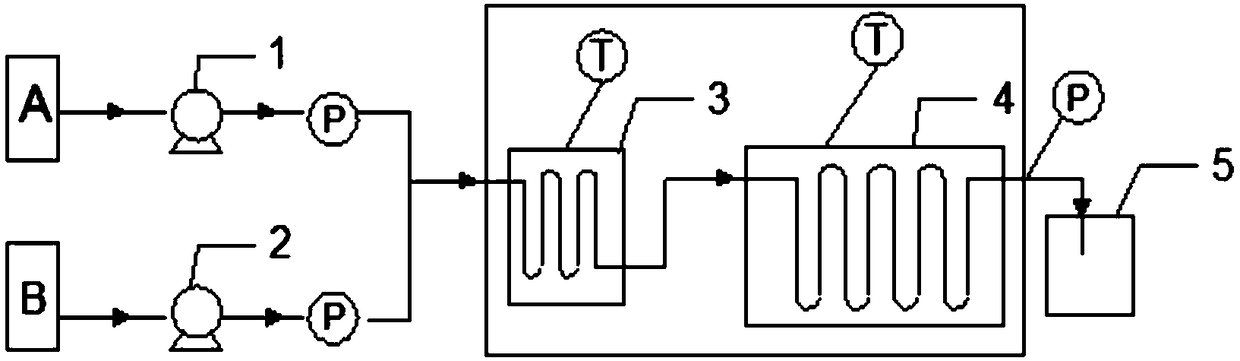
Method and specially used apparatus for ortho-dichlorobenzene continuous mononitration reaction
InactiveCN107417536APrecise control of reaction parametersOvercome problems such as uneven local concentrationProcess control/regulationChemical/physical/physico-chemical stationary reactorsNitrationMetering pump
The invention discloses a method and specially used apparatus for ortho-dichlorobenzene continuous mononitration reaction. The method is characterized in that raw materials are added into an ortho-dichlorobenzene metering tank; a nitrating agent A is added into a nitrating agent A metering tank; the raw materials and the nitrating agent A are separately input into a first mixer synchronously through a first metering pump and a second metering pump; a reaction is performed at a temperature of 100-200 DEG C for 15-50 min; a reaction solution A enters a first liquid separation device; after liquid separation, an organic phase A enters a second mixer and an inorganic phase A enters a waste acid receiving device; a nitrating agent B stored in a nitrating agent B metering tank is added into the second mixer through a third metering pump; a nitration reaction is performed in a second tube reactor at a temperature of 10-100 DEG C; a reaction solution B enters a second liquid separation device; after liquid separation, an inorganic phase B enters the waste acid receiving device and an organic phase B enters a product postprocessing device for obtaining a mononitration product. The concentration of sulfuric acid used in the preparation method provided by the invention is low, the raw material conversion is complete, the yield of the final product is high, multi-nitro by-products are less, and the safety is higher, so that the preparation method and the apparatus are suitable for industrialized production.
Owner:ZHEJIANG UNIV OF TECH
Method for simultaneously preparing octogen and hexogen
InactiveCN101863849ARaw materials are easy to obtainHigh yieldOrganic chemistryNitrated acyclic/alicyclic/heterocyclic amine explosive compositionsTemperature controlAnti solvent
The invention relates to a method for simultaneously preparing octogen and hexogen, and belongs to the field of applied chemistry. The method comprises the following steps of: condensing acetonitrile and trioxymethylene serving as raw materials in the presence of a catalyst concentrated sulfuric acid to obtain a mixture of TAT and TRAT; and nitrating the mixture of TAT and TRAT in the presence of nitrating agents, namely concentrated nitric acid and phosphorus pentoxide to obtain a mixture of HMX and RDX, purifying the HMX and the RDX by adopting a DMF complex separation method, and purifying the RDX by adopting an anti-solvent phase separation method; or separating the mixture of TAT and TRAT and then nitrating to obtain the HMX and the RDX. The method has the advantages of simple and readily available raw materials, high yield, low cost, mild nitrating condition, easy temperature control, high safety and small pollution; and the simultaneously obtained HMX and RDX nitro-amine dynamite at one time can be directly used for preparing dynamite formulas without separation.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
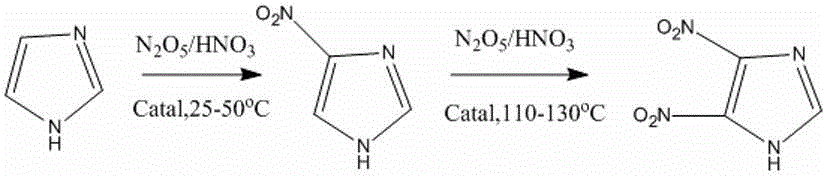
Preparation method of 4-nitroimidazole and 4,5-dimetridazloe
InactiveCN104892522AHigh synthesis efficiencyAvoid mixed acidPhysical/chemical process catalystsOrganic chemistryNitroimidazoleNitration
The invention relates to a preparation method of 4-nitroimidazole and 4,5-dimetridazloe, and in particular relates to a method for catalyzing a nitroimidazole compound by mesoporous SO4<2-> / ZrO2-CeO2 super acid. According to the method, a N2O5 / nitrosonitric acid saturated solution serves as a nitrating agent and super acid serves as a catalyst; imidazole and 4-nitroimidazole are added respectively; a reaction kettle is sealed after slurry reaction liquid is formed by stirring and mixing at normal temperature; reaction is performed respectively at the temperature of 25-50 DEG C and 110-130 DEG C until the raw materials are transformed completely; and the productive rates of 4-nitroimidazole and 4,5-dimetridazloe are 72-90% and 66-85% respectively. Compared with the existing literature, the method provided by the invention has the advantages of avoiding usage of mixed acid such as sulfuric acid and anhydride and improving the synthesis efficiency of the nitrating agent; and the product is easy to separate and the catalyst can be recycled, so that the method is an environmental-friendly green imidazole nitration process.
Owner:ZHONGBEI UNIV
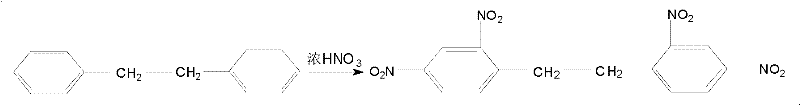
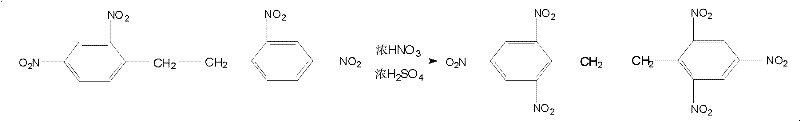
New method for synthesizing hexanitrobibenzyl
InactiveCN102399150AHigh yieldEasy to operateNitro compound preparationReaction temperatureNitration
The invention discloses a new method for synthesizing hexanitrobibenzyl, and relates to the technical field of preparation of energetic materials. The method comprises the following steps of: nitrating, separating out, filtering, washing, crystallizing and drying bibenzyl at the temperature of 18-35 DEG C to obtain tetranitrobibenzyl; further nitrating the tetranitrobibenzyl at the temperature of 80-120 DEG C; and separating out, then filtering, washing, crystallizing and drying to finally obtain a hexanitrobibenzyl pure product. In the method, by changing a nitrating agent and nitration reaction temperature, synthetic reaction from the bibenzyl to the tetranitrobibenzyl is finished at normal temperature, so that the yield of crude product is increased by over 5 percent; the synthetic reaction from the tetranitrobibenzyl to the hexanitrobibenzyl is finished in one step, so that the operation process is simplified, the reaction time is shortened by about 24 hours, the yield of the hexanitrobibenzyl pure product is increased by over 13 percent, the cost is reduced by about 20 percent, and the method is safe in operation and stable in reaction.
Owner:ZHONGBEI UNIV
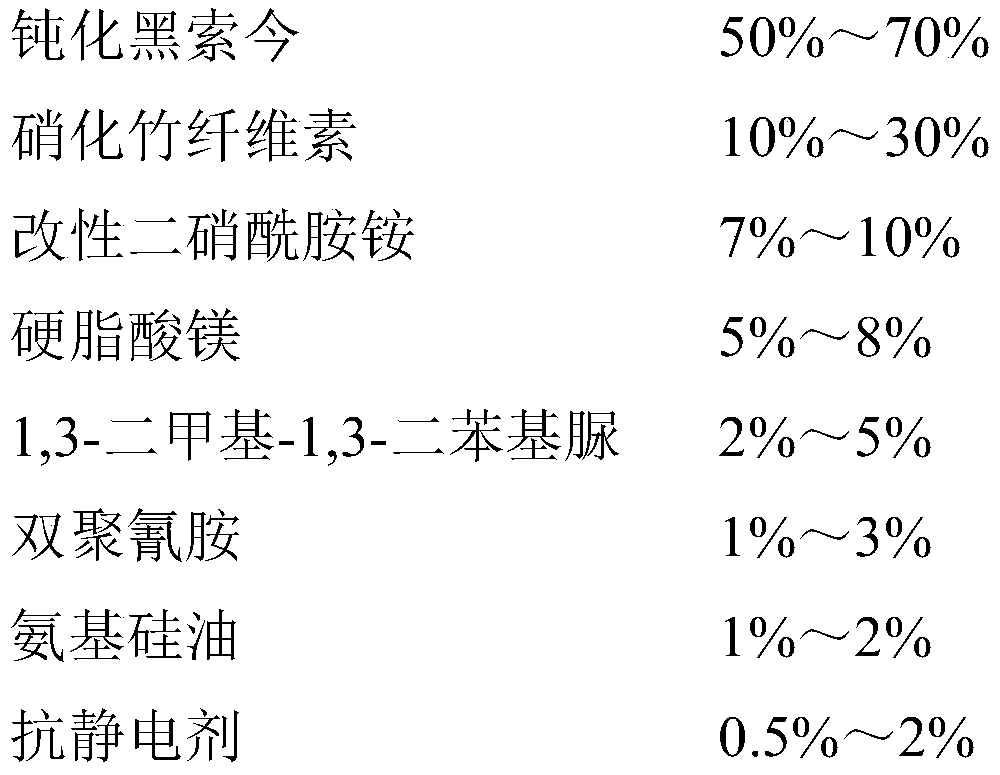
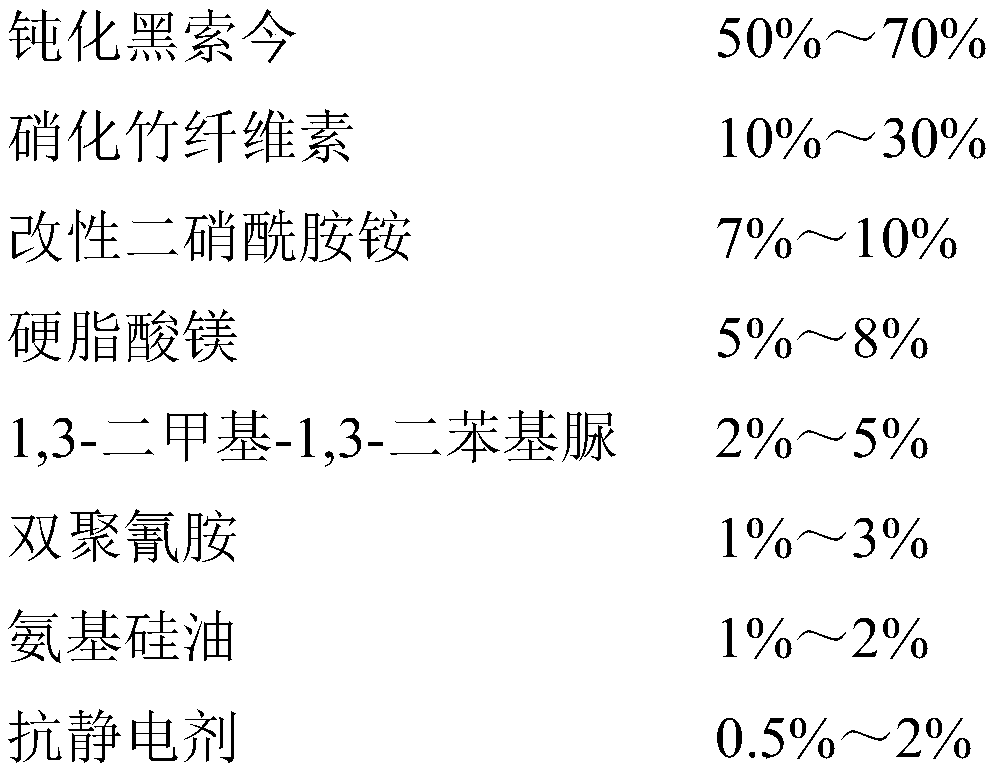
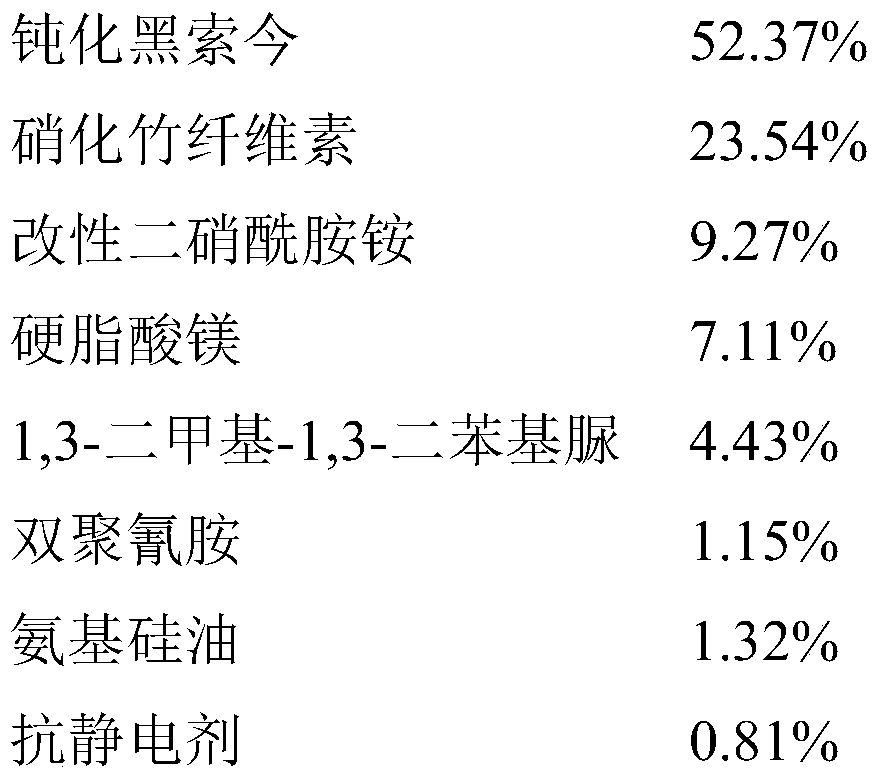
Antistatic smokeless sulfur-free firecracker nitrate agent and preparation method thereof
PendingCN110981657ASufficient oxygenGuaranteed combustion effectExplosive working-up apparatusNitrated acyclic/alicyclic/heterocyclic amine explosive compositionsCelluloseNitration
The invention relates to an antistatic smokeless sulfur-free firecracker nitrate agent and a preparation method thereof, belonging to the field of environment-friendly pyrotechnic compositions. The firecracker nitrate agent comprises, desensitized hexogen, nitrified bamboo cellulose, modified ammonium dinitramide, magnesium stearate, 1,3-dimethyl-1,3-diphenyl urea, dicyandiamide, amino silicon oiland an antistatic agent. According to the invention, desensitized hexogen is used as a main component, and modified ammonium dinitramide is used as an oxidant, so an oxygen content is sufficient, andcombustion effect is ensured; the nitrified bamboo cellulose, 1,3-dimethyl-1,3-diphenyl urea, magnesium stearate and dicyandiamide are adopted, and the antistatic agent and amino silicone oil are added, so the mechanical properties, stability and antistatic capacity of the firecracker nitrate agent can be remarkably improved, and the safety and reliability of the firecracker nitrate agent in theprocesses of production, storage and use are guaranteed; meanwhile, the firecracker nitrate agent does not contain a sulfur element or any metal powder, so the preparation of the smokeless sulfur-freefirecracker nitrate agent can be truly realized.
Owner:江西吉润花炮新材料科技有限公司
Method for preparing aromatic nitro compound using nitryl chloride as nitrating agent
The invention relates to a method for preparing an aromatic nitro compound by using nitryl chloride as a nitrating agent. In the method, the aromatic nitro compound is prepared by using tail gas of nitryl chloride as the nitrating agent, wherein a nitrated substance is aromatic hydrocarbon and benzene, chlorobenzene, nitrobenzene or toluene of derivatives of the aromatic hydrocarbon; the nitryl chloride is the nitrating agent and sulfuric acid is a medium; and the nitration is carried out on aromaticring at a temperature of between 20 and 90 DEG C at normal pressure. The method has the following advantages: (1) changing wastes to valuables, and providing economic benefit for companies; (2) saving water resource; and (3) having small investment on production devices and having smaller energy consumption compared with production devices in the same scale.
Owner:淮安嘉诚高新化工股份有限公司
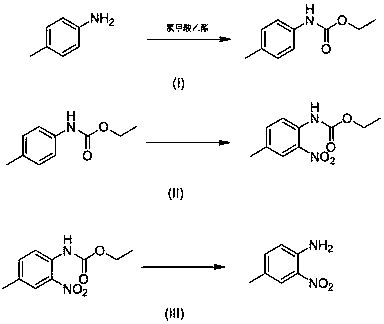
4-methyl-2-nitroaniline synthesis method
InactiveCN107759479AMild reaction conditionsNo need for high temperatureCarbamic acid derivatives preparationOrganic compound preparationNitrosoMethylaniline
The invention discloses a 4-methyl-2-nitroaniline synthesis method, which comprises: carrying out amino protection with ethyl chloroformate by using 4-methylaniline as a raw material to generate N-(p-toluene)ethyl carbamate; adding an oxidant and a copper salt catalyst, and carrying out a reaction for a certain time at a temperature of 50-120 DEG C in a reaction solvent by using a nitroso-containing compound as a nitrating agent to prepare a corresponding protected o-nitro-p-toluidine; and carrying out hydrolysis to obtain the target product. According to the present invention, the method hasadvantages of simple preparation process, mild reaction condition, high yield and environment protection.
Owner:NANJING UNIV OF SCI & TECH
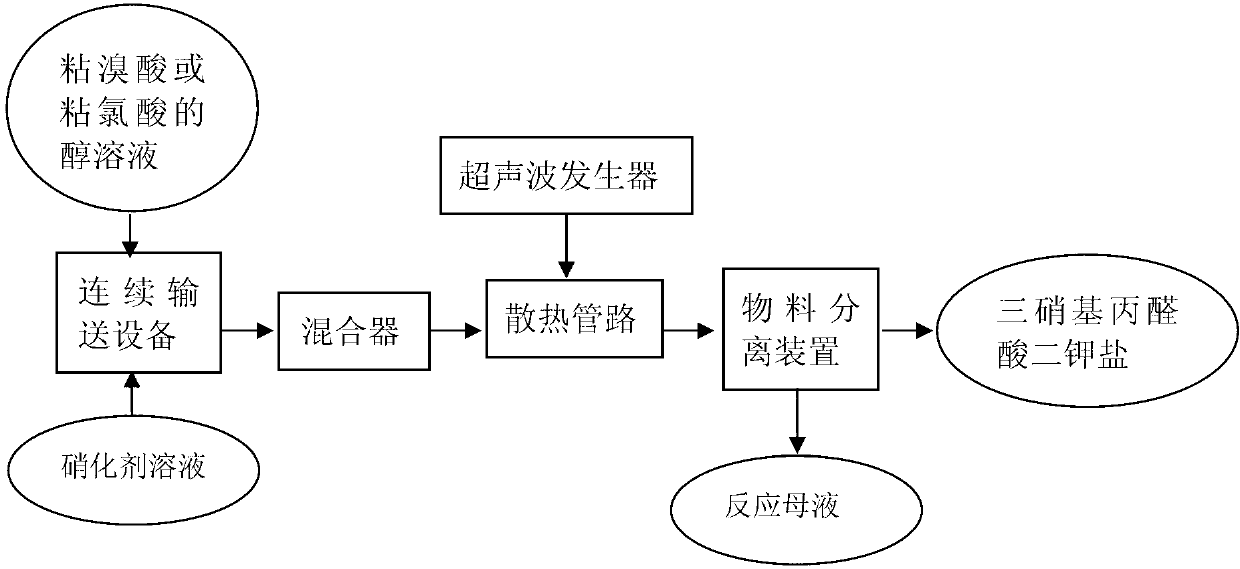
Continuous preparation method of trinitromalonaldehydic acid dipotassium salt
ActiveCN103342647ARealize the minimum online volumeAchieve separationOrganic chemistryOrganic compound preparationDistillationPotassium
The invention discloses a continuous preparation method of a trinitromalonaldehydic acid dipotassium salt. The continuous preparation method comprises the following steps of: conveying the alcoholic solution of mucobromic acid or mucochloric acid and a nitrating agent solution in parallel by using continuous conveying equipment and completing a reaction in a continuous reactor or a pipeline, performing ultrasonic dispersion treatment in the material conveying process, and carrying out the treatment processes such as solid-liquid separation, washing and drying on the generated product, thus obtaining the trinitromalonaldehydic acid dipotassium salt. Solvent distillation recovery is performed on the separated reaction mother liquor and thewashing liquid, and the distilled mother liquor is crystallized so that the by-product potassium bromide or potassium chloride can be separated out. The method is capable of improving the reaction efficiency, reducing the online handling capacity of materials and reducing or eliminating potential safety hazards probably caused by reaction heat release in the intermittent reaction process. Besides, the byproduct can be separated and utilized effectively; therefore, pollution on the environment can be avoided.
Owner:NANJING UNIV OF SCI & TECH
Preparation method of HMX
InactiveCN107286167AOvercome the shortcoming of extremely low yield of HMXOvercome the shortcoming of extremely low yieldOrganic chemistryAmmonium oxalateTreatment costs
The invention discloses a preparation method of HMX. The preparation method comprises the steps of dissolving dinitrogen pentoxide into an organic solvent to form a nitrating agent; slowly adding ammonium salt into the nitrating agent, and adding DPT in batches to obtain reactants; controlling the temperature of the materials to be 0-10 DEG C in a feeding process, heating the reactants up to 20-35 DEG C, and carrying out a reaction at the constant temperature for 20-60min; after the reaction is finished, carrying out solid-liquid separation to obtain solid; washing the obtained solid, drying in the air, and purifying to obtain the HMX, wherein the organic solvent is selected from acetonitrile and dichloromethane, and the ammonium salt is selected from tetramethyl ammonium chloride, ammonium carbonate, ammonium acetate and ammonium oxalate. The preparation method provided by the invention is mild in reaction conditions and easy in separation of products, and needs a less amount of acid; the system does not produce waste acid, thus being low in treatment cost; furthermore, the preparation method greatly increases the yield of the HMX.
Owner:WUHAN UNIV OF SCI & TECH
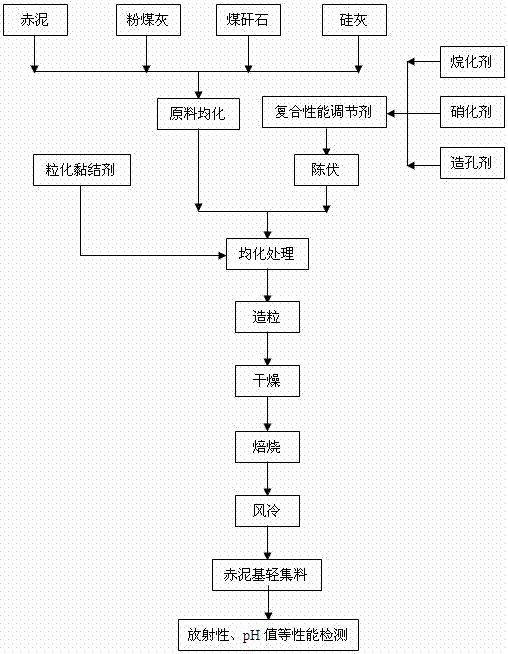
A kind of red mud-based sintered lightweight aggregate and preparation method thereof
ActiveCN105294142BSuppress radioactivityPlay a solid alkali effectSolid waste managementCeramicwareRed mudNitration
Owner:江西林宇建设工程有限公司
Method for synthesis of 2,4-dichloro-3,5-dinitro trifluorotoluene in micro reactor
InactiveCN106316859AReduce storage and transportationReduce riskNitro compound preparationNitrate agentTrifluorotoluene
The invention relates to a method for synthesis of 2,4-dichloro-3,5-dinitro trifluorotoluene in a micro reactor, that is to say, in the micro reactor, a raw material 2,4-dichloro trifluorotoluene and a nitrating agent composed of concentrated sulfuric acid and concentrated nitric acid are introduced in proportion for synthesis of 2,4-dichloro-3,5-dinitro trifluorotoluene. The method is simple, and is good in effect; compared with a traditional method, the method greatly shortens the reaction time (which is reduced from 96 h to about 200 s), improves the production efficiency, and has the 2,4-dichloro-3,5-dinitro trifluorotoluene yield of 40%.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing 1-methyl-4-nitropyrazole
InactiveCN107629003ARich sourcesNo pollution in the processOrganic chemistryNitrogen oxidesNitration
The invention provides a method for preparing 1-methyl-4-nitropyrazole. According to the invention, 1-methylpyrazole, N-nitropyrazole and a sulfuric acid solution are mixed and subjected to nitrationto obtain the 1-methyl-4-nitropyrazole. The method takes N-nitropyrazole and sulfuric acid as nitrated reagents, 1-methylpyrazole is directly nitrated to obtain the 1-methyl-4-nitropyrazole, the raw material source is abundant, the cost is low, usage of mixed acid of nitric acid and sulfuric acid as the nitrated agent is avoided, no polluted gas nitrogen oxide is generated during a reaction process, waste acid is not generated, environment pollution is little, furtherly, the reaction condition of the preparation method is mild, long time reaction at high temperature is not required, requirement on reaction equipment is low, purification is easy, and purity of the obtained product is high.
Owner:ZHONGBEI UNIV +1
N-methyl-4-nitrophthalimide preparation method
The invention relates to the technical field of organic synthesis processes, particularly to a N-methyl-4-nitrophthalimide preparation method, which comprises: preparation of a nitrating agent: mixinga nitrate and concentrated sulfuric acid to form a nitrating agent; preparation of N-methylphthalimide: adding methylamine and phthalic anhydride to a solvent, and carrying out heating reflux to obtain N-methylphthalimide; and preparation of N-methyl-4-nitrophthalimide: adding the nitrating agent to the N-methylphthalimide, and carrying out a reaction to form N-methyl-4-nitrophthalimide. According to the present invention, the nitrating agent is prepared at a low temperature by using nitrate and concentrated sulfuric acid so as to avoid the harm of fuming nitric acid to environmental and human body; and after the nitrating reaction is performed with the nitrating agent, the remaining nitrating agent can be recycled by extraction, and can further be added with an auxiliary agent to obtainnitrate or sulfate precipitate as a by-product, wherein ammonium nitrate or ammonium sulfate, and the like can be used as fertilizers so as to achieve environmentally friendly circulation reactions.
Owner:山东省鄄城县方源轴承化工有限公司
Preparing method of low-carbon nitroparaffins
The invention provides a preparing method of low-carbon nitroparaffins. According to the method, a reaction is carried out in a multi-passage heat insulation reaction vessel by using propane as raw materials and using nitric acid as nitrating agents; four kinds of target products are obtained through controlling the reaction temperature to be 300 to 450 DEG C, the pressure to be 0 to 1.0 Mpa, the dwell time to be 0.5 to 2s and the mol ratio of the propane to the nitric acid to be (2-6):1; the selectivity of nitromethane is 4 to 7 percent; the selectivity of nitroethane is 9 to 13 percent; the selectivity of 1-nitropropane is 40 to 45 percent; and the selectivity of 2-nitropropane is 35 to 40 percent. The preparing method of the low-carbon nitroparaffins provided by the invention mainly has two major advantages that (1) four kinds of nitroparaffins with specific selectivity can be simultaneously obtained; and (2) the selectivity of nitration products is adjustable in a certain range.
Owner:嘉兴润博化工科技有限公司
Process for the manufacture of nicorandil
Disclosed is a process for the synthesis of Nicorandil (1), 2-(nicotinamide)ethyl nitrate, starting from N-(2-hydroxyethyl)nicotinamide (15), using nitration with nitric acid in the presence of acetic anhydride Said synthesis method is particularly advantageous because it solves the safety problems involved in the use of nitric acid as nitrating agent, and allows a product with excellent yields and quality to be isolated.
Owner:PROCOS SPA
Use of aluminium nitrate in phenol derivative nitrofication
InactiveCN1939888AEasy to operateEasy to recycleNitro compound preparationAluminium nitrateNitrate agent
A nitrated product and its reactive condition are disclosed. The nitrated product is prepared by reacting nitrating agent nitrating aluminum with aromatic derivative containing phenol hydroxyl.
Owner:NANJING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com