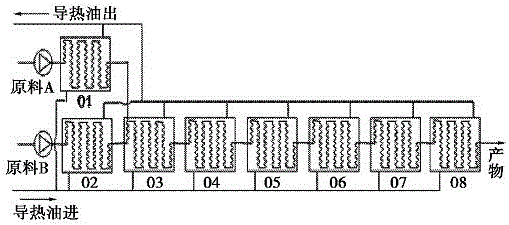
Method for synthesis of 2,4-dichloro-3,5-dinitro trifluorotoluene in micro reactor
A technology of dinitrotrifluorotoluene and dichlorotrifluorotoluene is applied in the field of organic fine synthesis, and achieves the effects of convenient operation, reduction of raw material storage, transportation and reaction risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The molar ratio of 95% nitric acid-98% sulfuric acid is 1:4, and the molar ratio of 2,4-dichlorotrifluorotoluene is 1.05. The two streams enter the microchannel for reaction, the residence time is 120s, and the reaction temperature is 140°C. The conversion rate of 2,4-dichlorobenzotrifluoride was 97.2%, and the yield of 2,4-dichloro-3,5-dinitrobenzotrifluoride was 22.6%.
Embodiment 2
[0025] The molar ratio of 95% nitric acid-98% sulfuric acid is 1:5, and the molar ratio of 2,4-dichlorotrifluorotoluene is 1.05. The two streams enter the microchannel for reaction, the residence time is 120s, and the reaction temperature is 150°C. The conversion rate of 2,4-dichlorobenzotrifluoride was 97.7%, and the yield of 2,4-dichloro-3,5-dinitrobenzotrifluoride was 30.8%.
Embodiment 3
[0027] The molar ratio of 95% nitric acid-98% sulfuric acid is 1:6, and the molar ratio of 2,4-dichlorotrifluorotoluene is 1.05. The two streams enter the microchannel for reaction, the residence time is 150s, and the reaction temperature is 160°C. The conversion rate of 2,4-dichlorobenzotrifluoride was 98.1%, and the yield of 2,4-dichloro-3,5-dinitrobenzotrifluoride was 40.7%.
[0028] In the above embodiment, the reaction time is only about 200s, the operation is simple and the effect is good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com