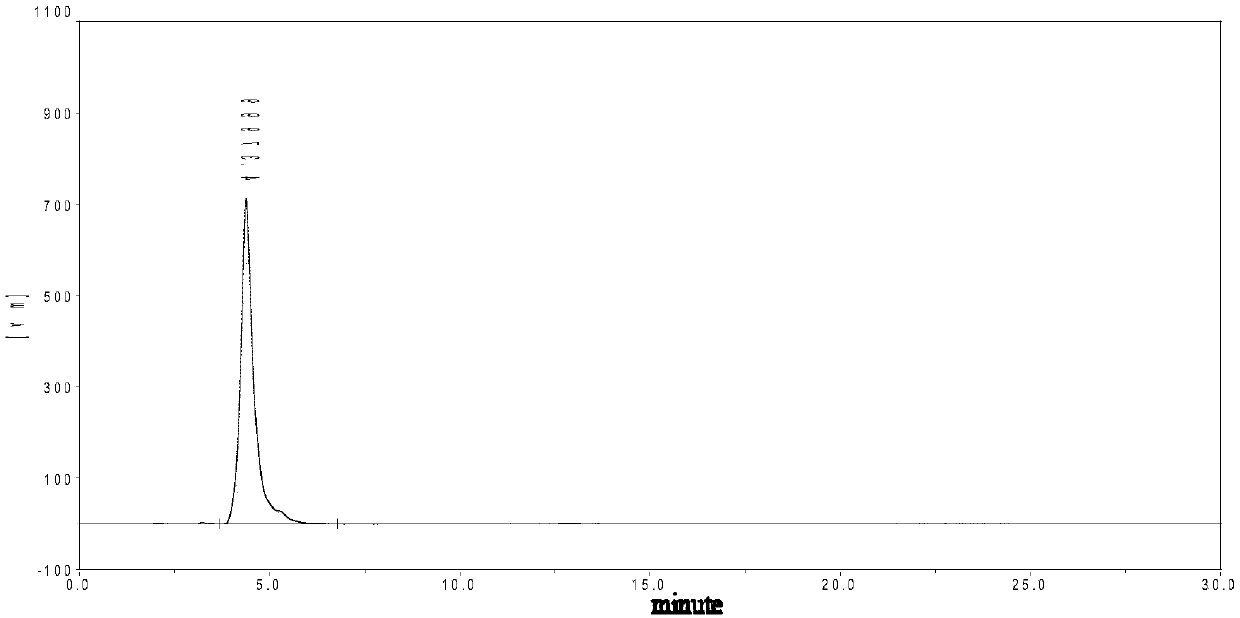
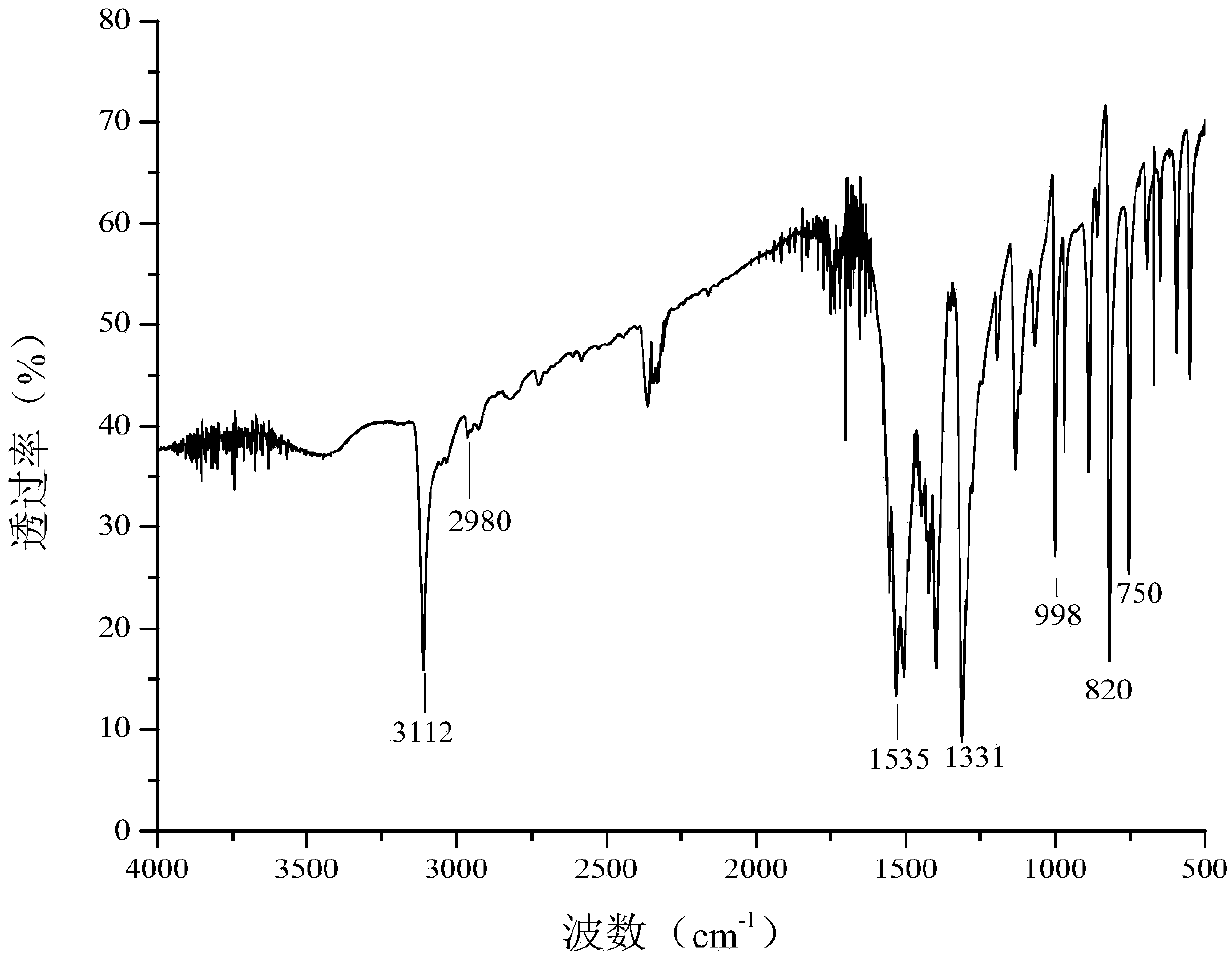
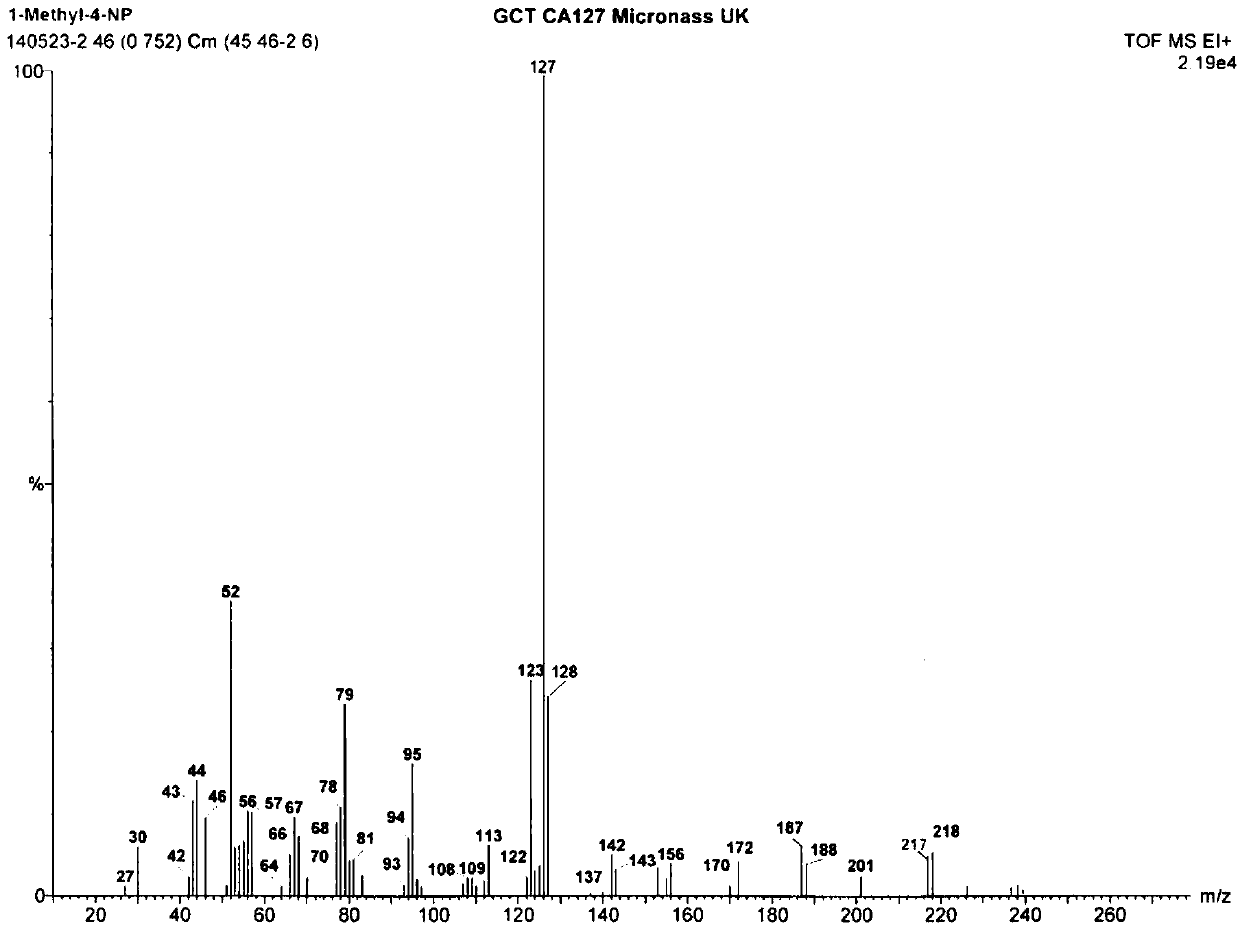
Method for preparing 1-methyl-4-nitropyrazole
A technology for nitropyrazole and methylpyrazole, which is applied in the field of preparation of 1-methyl-4-nitropyrazole, can solve the problems of pollution, excessive waste acid environment, a large amount of nitrogen oxide gas and the like, and achieves Abundant sources of raw materials, less environmental pollution, and low requirements for reaction equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The invention provides a kind of preparation method of 1-methyl-4-nitropyrazole, comprises the following steps:
[0031] Mix 1-methylpyrazole, N-nitropyrazole and sulfuric acid solution for nitration reaction to obtain 1-methyl-4-nitropyrazole.
[0032] In the present invention, the molar ratio of the 1-methylpyrazole, N-nitropyrazole and sulfuric acid in the sulfuric acid solution is preferably 1:1~3:7.5~12.5, more preferably 1:1.5~2.5 : 9~11; The mass concentration of described sulfuric acid solution is preferably 40%~98%, more preferably 75%~90%, most preferably 85~90%. In the present invention, sulfuric acid can protonate the unsubstituted nitrogen atom of N-nitropyrazole, so that the nitro group on its adjacent nitrogen atom is activated to produce nitroxyl ion (NO 2 + ), nitric oxide ion (NO 2 + ) further undergoes C-nitration reaction with N-methylpyrazole.
[0033] In the present invention, the temperature of the nitration reaction is preferably 20-80° C., ...
Embodiment 1
[0055] Take 1.4g (0.012mol) of N-nitropyrazole and 1g (0.012mol) of 1-methylpyrazole respectively for later use, add 8mL of 98% concentrated sulfuric acid into a 100mL four-necked bottle equipped with mechanical stirring, a thermometer, and an addition funnel 1-Methylpyrazole was added dropwise to concentrated sulfuric acid at 20°C with an addition funnel, and the water bath was heated to 60°C after the addition was completed. At this temperature, N-nitropyrazole was added in 10 times, and the amount added each time was 0.0012mol, the feeding is completed, keep stirring and reacting at constant temperature for 6 hours, after the reaction is completed, pour the reaction solution into a beaker with ice cubes while it is hot and stir, white fine crystalline solids are precipitated, the ice cubes are completely melted, suction filtration, and white Solid I and filtrate; the filtrate was extracted with ether, the solvents were combined, and the solvent was removed by rotary evaporat...
Embodiment 2
[0070] Take 4.1g (0.036mol) of N-nitropyrazole and 1g (0.012mol) of 1-methylpyrazole respectively for later use, and add 6mL of 98% concentrated sulfuric acid into a 100mL four-port container equipped with mechanical stirring, a thermometer, and an addition funnel In the bottle, add 1-methylpyrazole dropwise to the concentrated sulfuric acid with a constant pressure dropping funnel at 20°C. After the addition, the water bath is heated to 80°C. At this temperature, N-nitropyrazole is added in 12 times. Add 0.003mol at a time. After the addition is complete, react at a constant temperature of 20°C for 4 hours. Pour the reaction solution into a beaker filled with ice cubes while it is hot and stir. White fine crystalline solids precipitate out. After the ice cubes are completely melted, filter them with suction. A white solid I was obtained; the filtrate was extracted with ether, the solvents were combined, and the solvent was removed by rotary evaporation under reduced pressure t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com