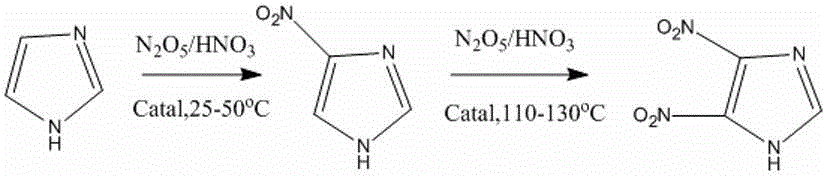
Preparation method of 4-nitroimidazole and 4,5-dimetridazloe
A technology of dinitroimidazole and nitroimidazole is applied in chemical recycling, organic chemistry and other directions to achieve the effect of improving synthesis efficiency and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] Example 1: A kind of synthetic method of 4-nitroimidazole
[0022] Step 1. In a polytetrafluoroethylene-lined stainless steel reactor, the N 2 o 5 Dissolves in fuming nitric acid to form N 2 o 5 / fuming nitric acid saturated solution, followed by adding mesoporous SO 4 2- / ZrO 2 -CeO 2 The superacid catalyst and imidazole are stirred and mixed at room temperature to form a slurry reaction liquid, wherein the molar ratio of imidazole to fuming nitric acid is 1 / 2, and the amount of catalyst is 10% of the mass of fuming nitric acid;
[0023] Step 2. Seal the reaction kettle tightly, raise the temperature to 25°C, and stir until the reaction is complete;
[0024] Step 3, cooling and diluting the reaction solution in step 2 in ice water, standing, layering, and filtering to recover the catalyst, extracting and filtering the reaction solution with dichloromethane, taking the organic phase, rotary evaporation, and drying to obtain 4-nitroimidazole , the yield is 72%, ...
example 2
[0025] Example 2: A kind of synthetic method of 4-nitroimidazole
[0026] Step 1. In a polytetrafluoroethylene-lined stainless steel reactor, the N 2 o 5 Dissolves in fuming nitric acid to form N 2 o 5 / fuming nitric acid saturated solution, followed by adding mesoporous SO 4 2- / ZrO 2 -CeO 2 The superacid catalyst and imidazole are stirred and mixed at room temperature to form a slurry reaction liquid, wherein the molar ratio of imidazole to fuming nitric acid is 1 / 3, and the amount of catalyst used is 10% of the mass of fuming nitric acid.
[0027] Step 2. Seal the reaction kettle tightly, raise the temperature to 30°C, and stir until the reaction is complete;
[0028] Step 3, cooling and diluting the reaction solution in step 2 in ice water, standing, layering, and filtering to recover the catalyst, extracting and filtering the reaction solution with dichloromethane, taking the organic phase, rotary evaporation, and drying to obtain 4-nitroimidazole , the yield is ...
example 3
[0029] Example 3: A kind of synthetic method of 4-nitroimidazole
[0030] Step 1. In a polytetrafluoroethylene-lined stainless steel reactor, the N 2 o 5 Dissolves in fuming nitric acid to form N 2 o 5 / fuming nitric acid saturated solution, followed by adding mesoporous SO 4 2- / ZrO 2 -CeO 2 The superacid catalyst and imidazole are stirred and mixed at room temperature to form a slurry reaction liquid, wherein the molar ratio of imidazole to fuming nitric acid is 1 / 4, and the amount of catalyst used is 15% of the mass of fuming nitric acid.
[0031] Step 2. Seal the reactor, heat up to 50°C, and stir until the reaction is complete;
[0032] Step 3, cooling and diluting the reaction solution in step 2 in ice water, standing, layering, and filtering to recover the catalyst, extracting and filtering the reaction solution with dichloromethane, taking the organic phase, rotary evaporation, and drying to obtain 4-nitroimidazole , the yield is 90%, the purity is 95%, the me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com