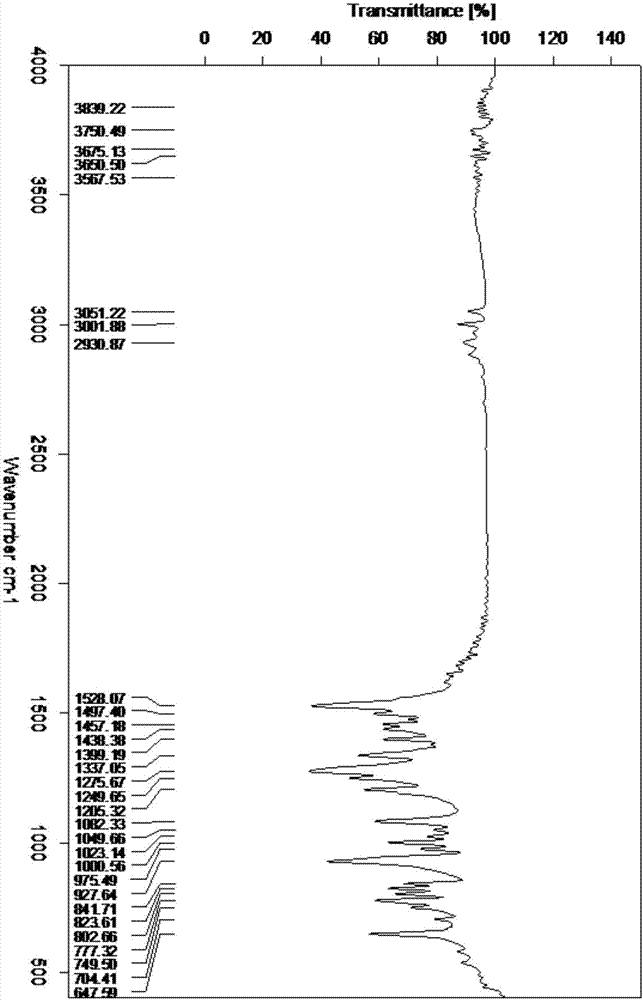
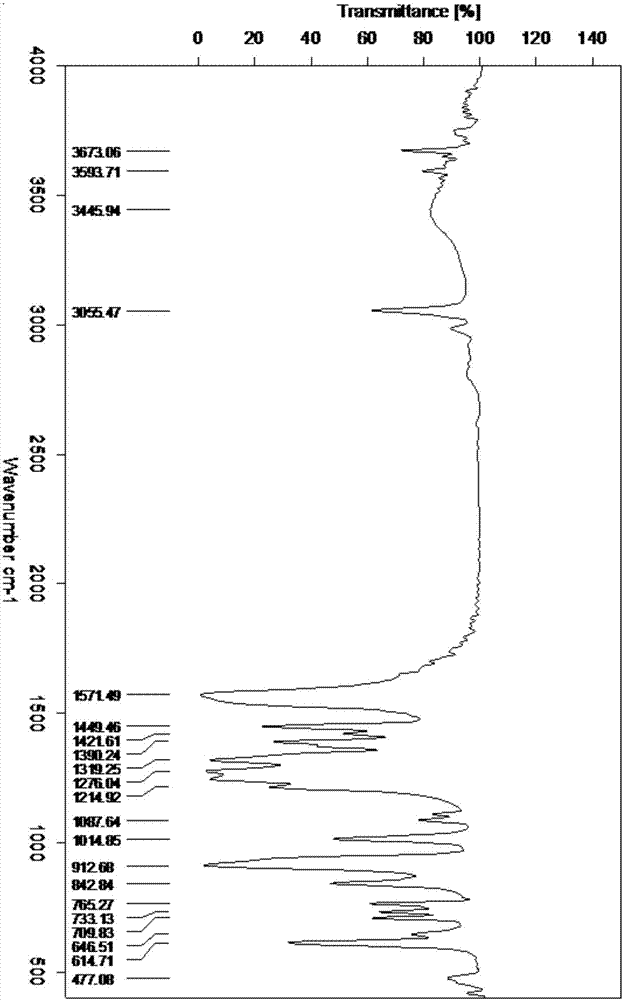
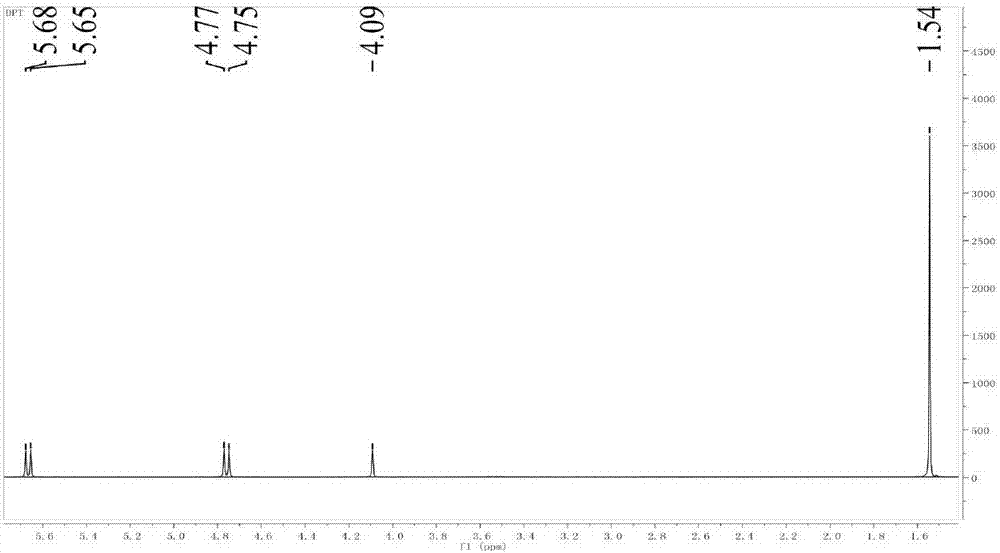
Preparation method of HMX
A technology of tetraazacyclooctane and tetraazabicyclo, which is applied in the field of energetic material synthesis and green nitration, can solve the problems of low yield and impractical application, and overcome the extremely low yield and low post-processing cost , The effect of high process atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1,3,5,7-tetranitro-1,3,5,7-tetraazacycloctane preparation method of the present invention, the method comprises: dissolving dinitrogen pentoxide in an organic solvent to form a nitrated agent, ammonium salt is slowly added to the nitrating agent, and 3,7-dinitro-1,3,5,7-tetraazabicyclo[3,3,1]nonane (DPT) is added in batches to obtain For the reactant, the temperature of the material is controlled at 0-10°C during the feeding process, the temperature of the reactant is raised to 20-35°C, and the reaction is carried out at a constant temperature for 20-60min; after the reaction is completed, the solid-liquid is separated to obtain a solid, and the solid is washed. Dried, purified to obtain 1,3,5,7-tetranitro-1,3,5,7-tetraazacyclooctane (HMX);
[0025] The organic solvent is selected from acetonitrile and dichloromethane, and the ammonium salt is selected from tetramethylammonium chloride, ammonium carbonate, ammonium acetate and ammonium oxalate.
[0026] The raw...
Embodiment 2
[0041] The preparation method of 1,3,5,7-tetranitro-1,3,5,7-tetraazacyclooctane in Example 2 of the present invention comprises the following steps:
[0042] Step 1, the preparation of nitrourea
[0043] Add 540ml of concentrated sulfuric acid with a concentration of 98% to a 1000ml three-necked flask under an ice-salt bath, weigh 175g of dry urea, and slowly add it into the three-necked flask under vigorous stirring. Concentration is slowly added dropwise in the flask and is the concentrated nitric acid 130ml of 68% (whole process control temperature is under 10 ℃). After the concentrated nitric acid is added, the temperature is lowered to below 5°C, and the reaction is kept for 90 minutes. After the reaction is completed, slowly add the reaction solution in the three-neck flask to two beakers with 500g of ice water under stirring conditions, and control the temperature not to exceed 15°C. During this period, white solids will be precipitated, and then suction filtered A wh...
Embodiment 3
[0049] The preparation method of 1,3,5,7-tetranitro-1,3,5,7-tetraazacycloctane in Example 3 of the present invention comprises the following steps:
[0050] Step 1, the preparation of nitrourea
[0051] Add 540ml of concentrated sulfuric acid with a concentration of 98% to a 1000ml three-necked flask under an ice-salt bath, weigh 185g of dry urea, and slowly add it to the three-necked flask under vigorous stirring. Concentration is slowly added dropwise in the flask and is the concentrated nitric acid 140ml of 68% (whole process control temperature is under 10 ℃). After adding the concentrated nitric acid, lower the temperature to below 5°C and keep the reaction for 60 minutes. After the reaction is completed, slowly add the reaction solution in the three-neck flask to two beakers with 500g of ice water under stirring conditions, and control the temperature not to exceed 15°C. During this period, white solids will be precipitated, and then suction filtered A white solid - ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com