Preparation method of trienes liquid crystal monomers
A technology of liquid crystal monomers and olefins, applied in the field of preparation of triene liquid crystal monomers, can solve the problems of unfavorable industrial production and low product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
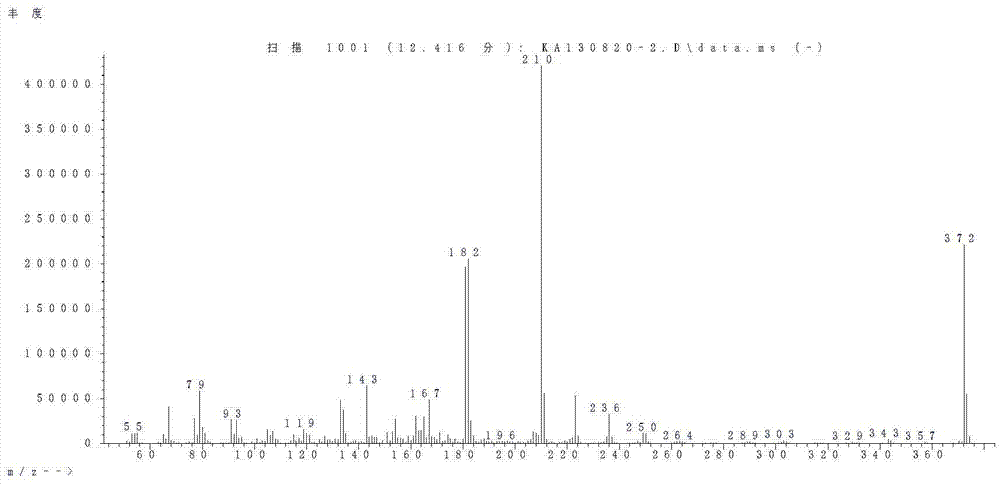
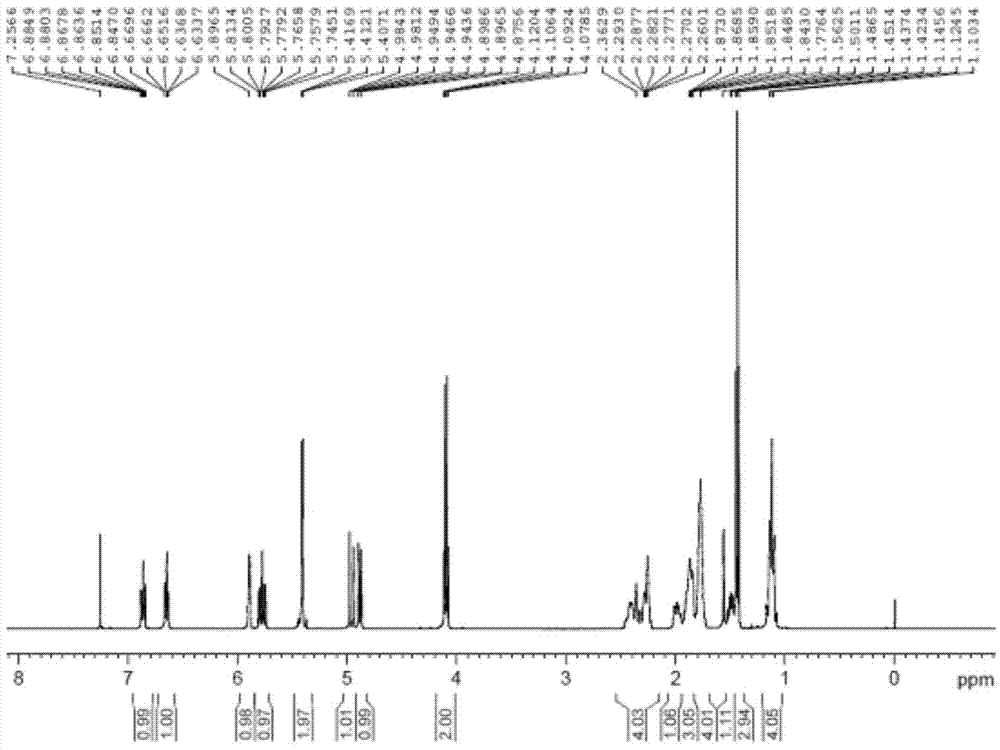
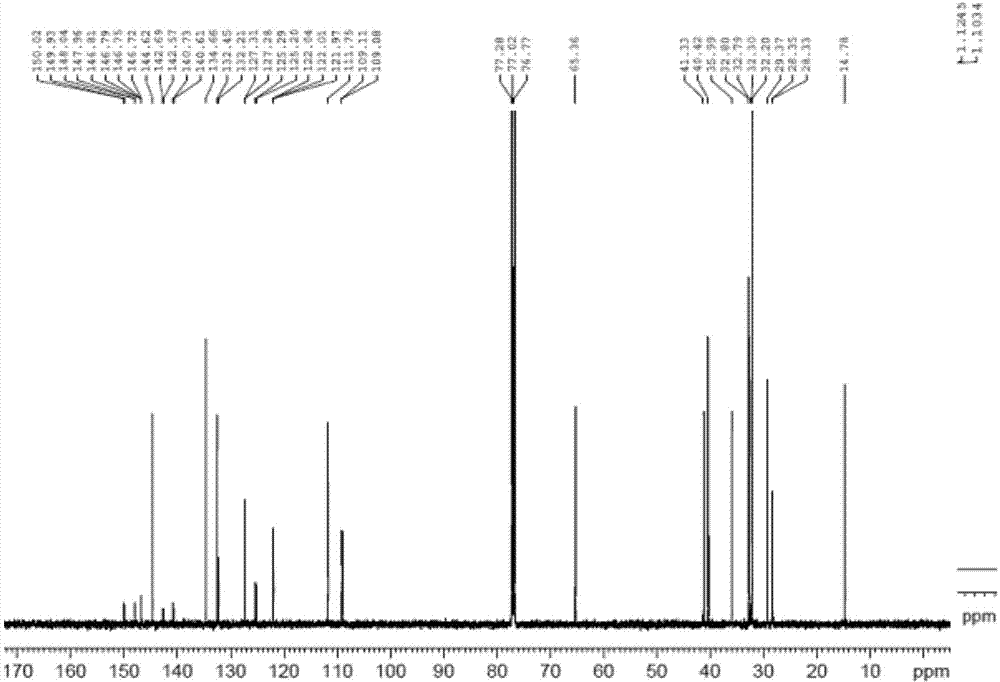
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of 1-ethoxy-2,3-difluoro-4-{4-[2-(4-vinyl-cyclohexyl)-vinyl]-cyclohex-1-enyl}-benzene
[0062] 1) 4-(4-ethoxy-2,3-difluoro-phenyl)-4-hydroxy-cyclohexanone ethylene acetal Put 99.5g (0.63mol) of 1-ethoxy-2,3-difluorobenzene and 600g of THF into a 2L three-necked flask, stir and cool the system down to -60°C, and dropwise add 286mL (0.63mol) of n-butyllithium n-hexane solution, keep warm at -60°C for 2h, add dropwise a solution of 93.6g (0.6mol) KCR and 150g THF, keep warm at -60°C for 1h, hydrolyze and separate layers, wash the organic phase with water until pH = 7, do not do any treatment, and wait for the next step Reaction, product GC purity 96.2%.
[0063] 2) 4-(4-ethoxy-2,3-difluoro-phenyl)-4-hydroxy-cyclohexanone In the 2L there-necked flask, drop into the solution of 4-(4-ethoxy-2,3-difluoro-phenyl)-4-hydroxyl-cyclohexanone ethylene ketal obtained in the previous step, 324g of formic acid with a mass fraction of 85% , 60 ℃ heat preservat...
Embodiment 2
[0070] Preparation of 1-butoxy-2,3-difluoro-4-{4-[2-(4-vinyl-cyclohexyl)-vinyl]-cyclohex-1-enyl}-benzene
[0071] 1) 4-(4-Butoxy-2,3-difluoro-phenyl)-4-hydroxy-cyclohexanone ethylene acetal Put 15.1g (0.63mol) of magnesium bars into a 2L three-necked flask, add dropwise a solution of 158.4g (0.63mol) of 1-bromo-4-butoxy-2,3-difluorobenzene and 500g of THF at 25°C, and react 2 After 1 hour, a solution of 93.6g (0.6mol) KCR and 150g toluene was added dropwise, kept at 25°C for 1h, hydrolyzed and separated, and the organic phase was washed with water until pH = 7, then left untreated and waited for the next reaction. The GC purity of the product was 93.8%.
[0072] 2) 4-(4-butoxy-2,3-difluoro-phenyl)-4-hydroxy-cyclohexanone Put the solution of 4-(4-butoxyl-2,3-difluoro-phenyl)-4-hydroxyl-cyclohexanone ethylene ketal obtained in the previous step into a 2L three-necked flask, 268.2g of 85% mass fraction Formic acid, heat preservation reaction at 70°C for 2 hours; after heat pr...
Embodiment 3
[0079] Preparation of 1-ethyl-2,3-difluoro-4-{4-[2-(4-propenyl-cyclohexyl)-vinyl]-cyclohex-1-enyl}-benzene
[0080] 1) 4-(4-Ethyl-2,3-difluoro-phenyl)-4-hydroxy-cyclohexanone ethylene acetal Put 89.5g (0.63mol) of 1-ethyl-2,3-difluorobenzene and 400g THF into a 2L three-necked flask, stir and cool the system down to -40°C, add 0.63mol of sec-butyl lithium in n-hexane dropwise, Incubate at -40°C for 2 hours, add dropwise a solution of 93.6g (0.6mol) KCR and 150g THF, incubate at -40°C for 1h, hydrolyze and separate layers, wash the organic phase with water until pH = 7, leave it alone, wait for the next reaction, the product GC 97.1% purity.
[0081] 2) 4-(4-Ethyl-2,3-difluoro-phenyl)-4-hydroxy-cyclohexanone In the 2L there-necked flask, drop into the solution of 4-(4-ethyl-2,3-difluoro-phenyl)-4-hydroxyl-cyclohexanone ethylene ketal obtained in the previous step, 381g of formic acid with a mass fraction of 85%, Insulate and react at 60°C for 3 hours; complete the incubati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com