Patents
Literature
91results about "Ether preparation by isomerisation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
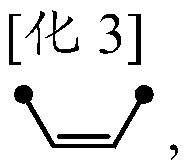
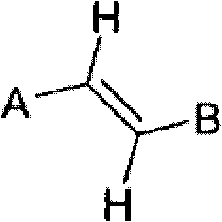
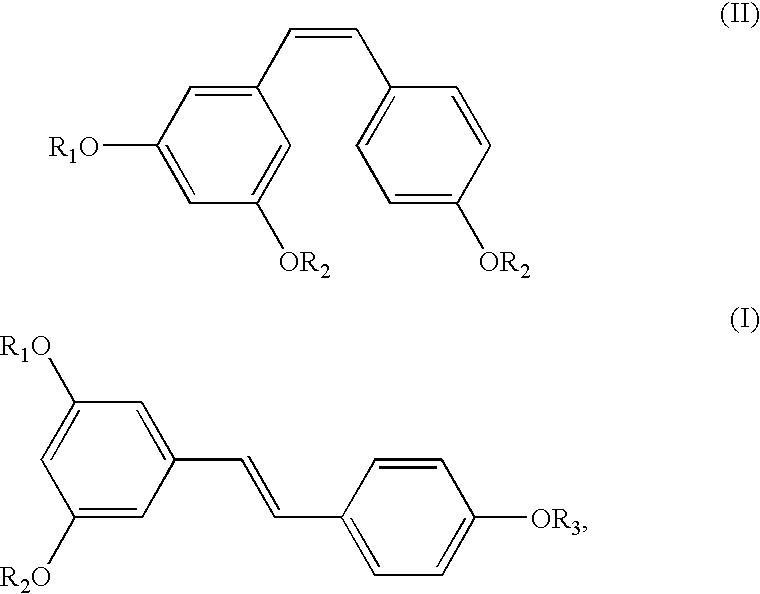
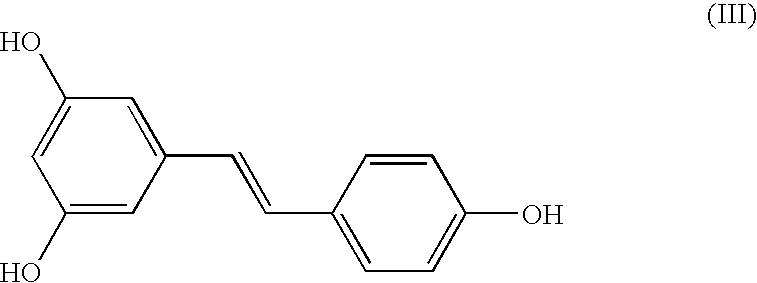
Method for the conversion of a Z-isomer into E-isomer
InactiveUS20040015020A1Simple processOrganic compound preparationOrganic chemistry methodsArylSilylene
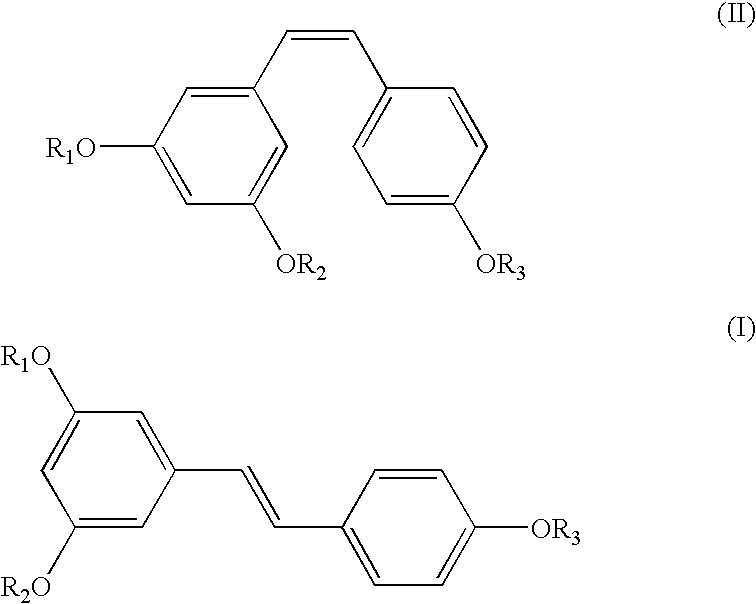
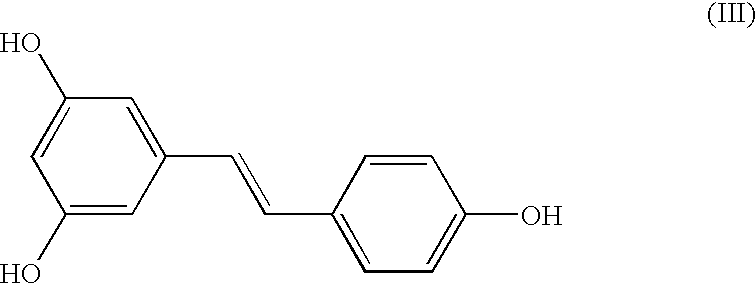
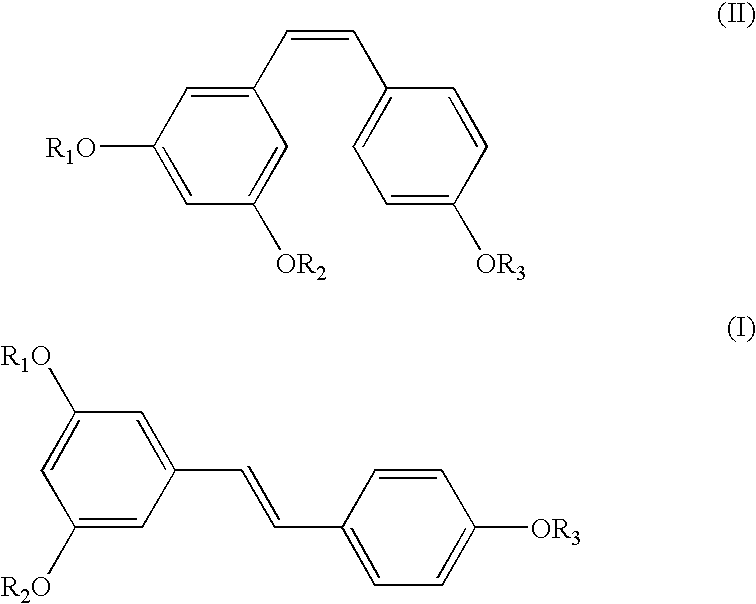
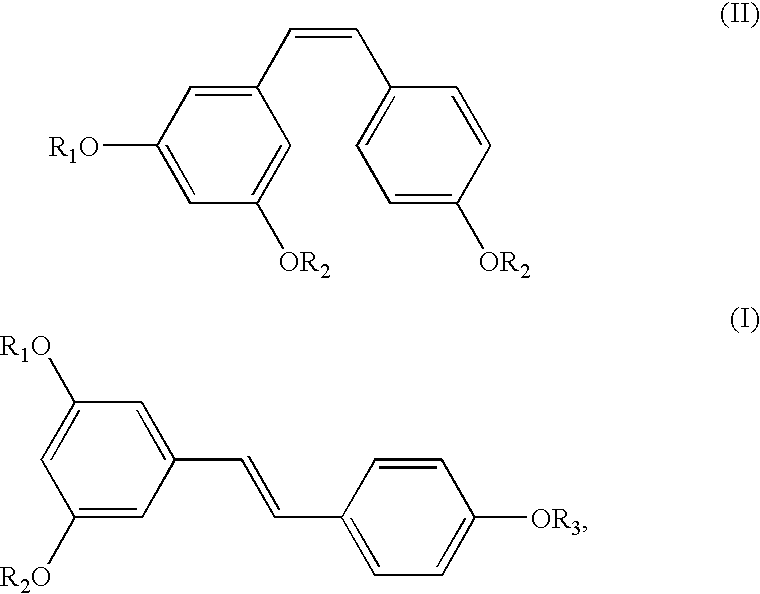
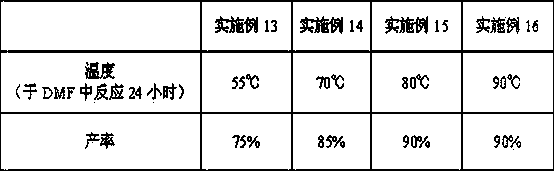
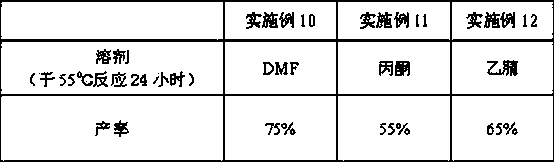
A method of converting (Z)-1-(3,5-disubstituted phenyl)-2-(4-substituted phenyl)ethene of general formula (II) to (E)-1-(3,5-disubstituted phenyl)-2-(4-substituted phenyl)ethene of general formula (I) wherein R1, R2 and R3 are same or different and independently represent (C1-C4)alkyl, (C1-C4)alkoxy(C1-C4)alkyl, (C1-C4)alkoxy(C1-C4)alkoxy(C1-C4)alkyl, allyl, vinyl, silyl, formyl, acyl, aryl(C1-C4)alkyl or substituted aryl(C1-C4)alkyl group. The present invention also provides a process for the conversion of (E)-1-(3,5-disubstituted phenyl)-2-(4-substituted phenyl)ethene of general formula (I) prepared by a process described above to E-resveratrol of the formula (III).
Owner:ORCHID CHEM & PHARM LTD
Diarylalkanes as potent inhibitors of binuclear enzymes
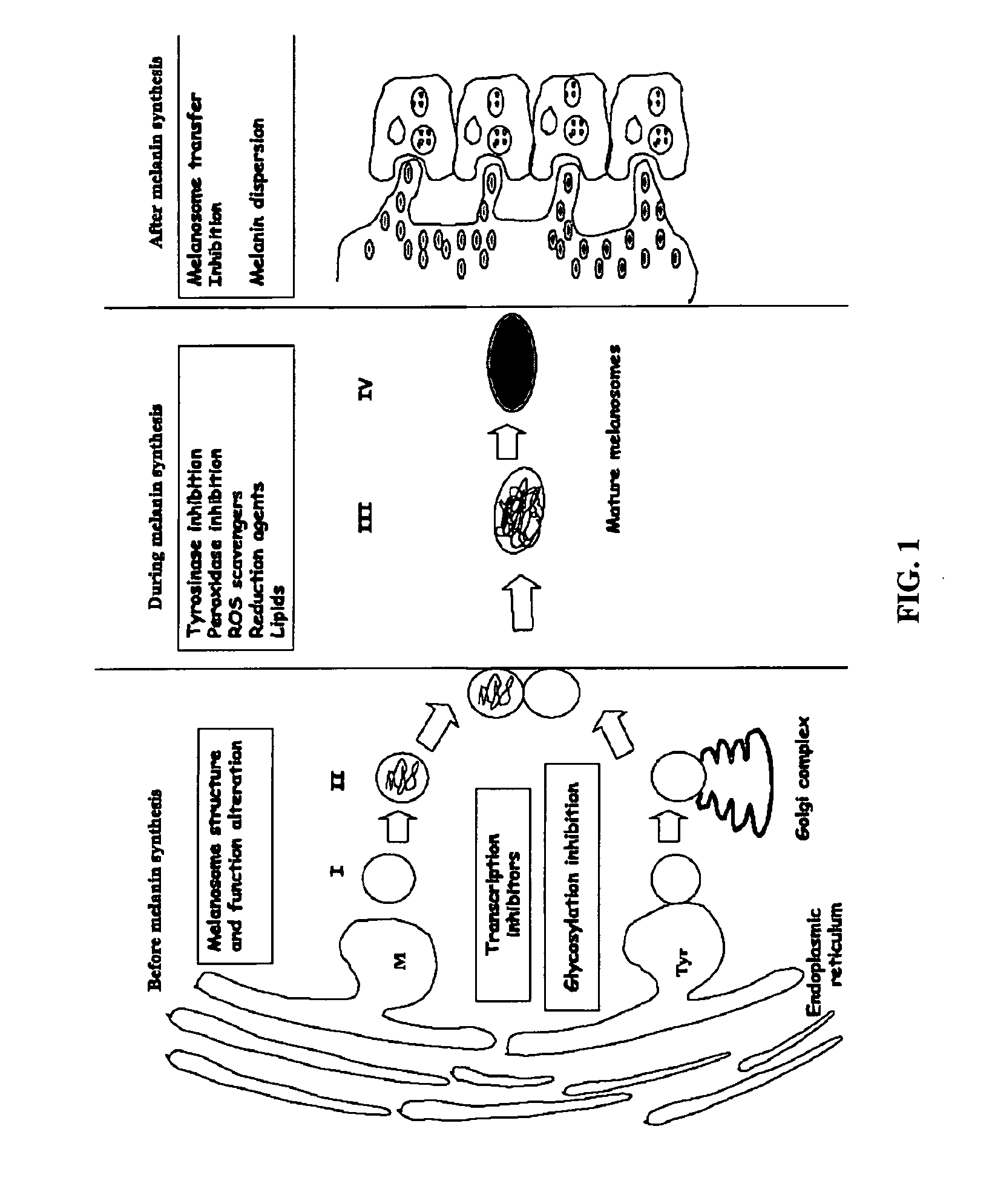
ActiveUS20080132581A1Avoid damagePrevent skinAntibacterial agentsOrganic active ingredientsDiseaseEnzyme inhibition
The present invention implements a strategy that combines an enzyme inhibition assay with a chemical dereplication process to identify active plant extracts and the particular compounds—diarylalkanes and / or diarylalkanols within those extracts that specifically inhibit binuclear enzyme function. Included in the present invention are compositions of matter comprised of one or more of diarylalkanes and / or diarylalkanols, which inhibit the activity of binuclear enzymes, particularly tyrosinase and which prevent melanin overproduction. The present invention also provides a method for inhibiting the activity of a binuclear enzyme, particularly tyrosinase and a method for preventing and treating diseases and conditions related to binuclear enzyme function. The present invention further includes a method for preventing and treating melanin overproduction and diseases and conditions of the skin related thereto. The method for preventing and treating diseases and conditions related to binuclear enzyme function and melanin overproduction is comprised of administering to a host in need thereof an effective amount of a composition comprising one or more diarylalkanes and / or diarylalkanols synthesized and / or isolated from one or more plants together with a pharmaceutically acceptable carrier.
Owner:UNIGEN
Porous microcomposite of metal cation exchanged perfluorinated ion-exchange polymer and network of metal oxide, silica, or metal oxide and silica
InactiveUS6034290AIncrease surface areaImprove accessibilityHydrocarbon by isomerisationCarboxylic acid esters preparationSide chainIon exchange
Porous microcomposites comprising a perfluorinated ion-exchange polymer (PFIEP) containing pendant metal cation exchanged sulfonate groups, metal cation exchanged carboxylate groups, or metal cation exchanged sulfonate and carboxylate groups, wherein the metal cation may be ligand coordinated, and optionally pendant sulfonic acid groups, carboxylic acid groups, or sulfonic acid and carboxylic acid groups, the PFIEP being entrapped within and highly dispersed throughout a network of metal oxide, a network of silica or a network of metal oxide and silica can be prepared from PFIEP and one or more precursors selected from the group consisting of a metal oxide precursor, a silica precursor, and a metal oxide and silica precursor using an in situ process. Preferred metal cations are Cr, Sn, Al, Fe, Os, Co, Zn, Hg, Li, Na, Cu, Pd or Ru. Such microcomposites have a first set of pores having a pore size diameter ranging from about 0.5 nm to about 75 nm and may further comprise a second set of pores having a diameter ranging from about 75 nm to about 1000 nm. These microcomposites possess high surface area and exhibit high catalytic activity. The catalyst is used to isomerize 1,4-dichloro-2-butene to 3,4-dichloro-1-butene, a precursor to neoprone.
Owner:EI DU PONT DE NEMOURS & CO
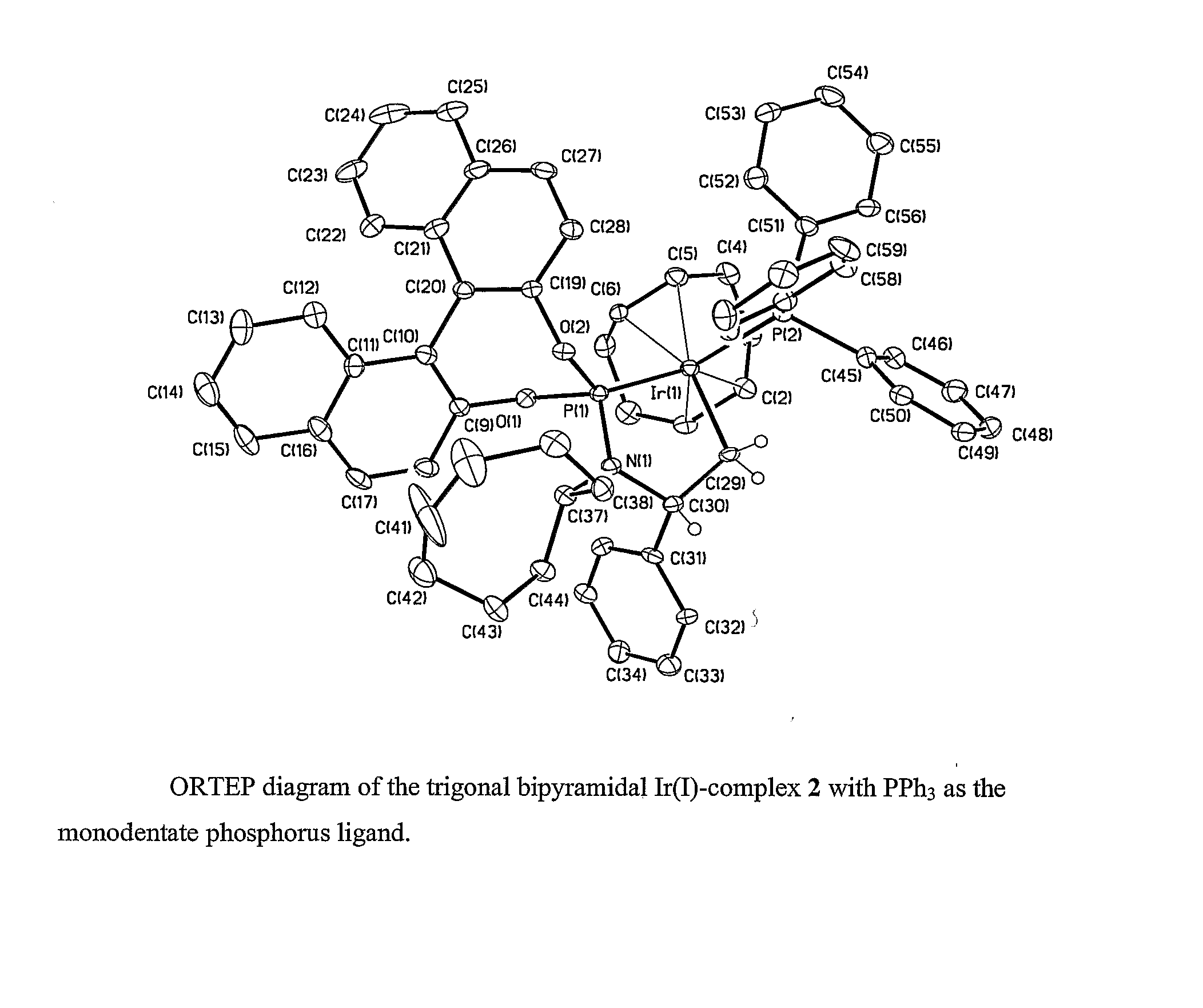
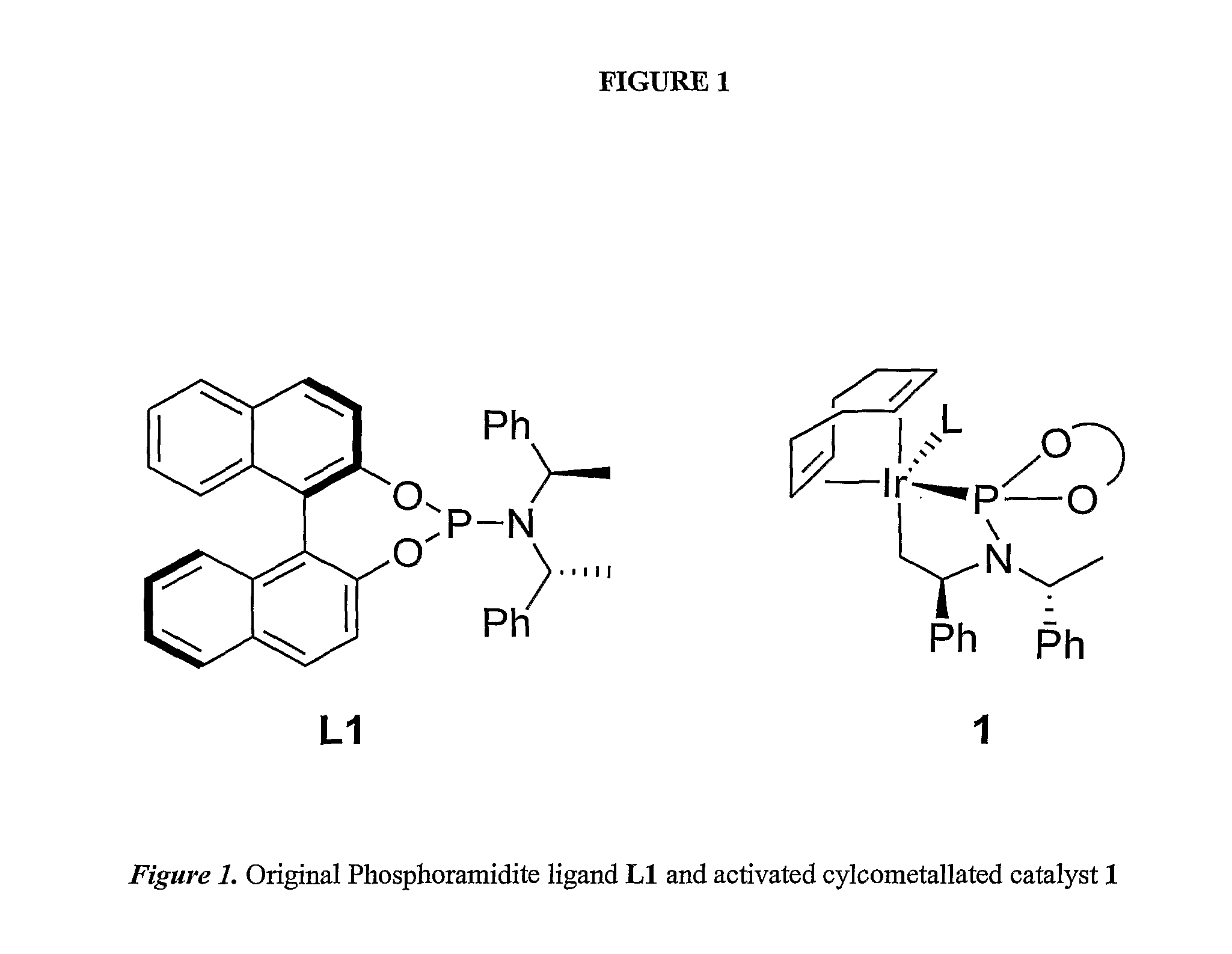
Enantioselective Phosphoramidite Compounds and Catalysts
InactiveUS20070259774A1Easy to getWithout compromising activity and degree of chemical selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystEnantio selectivity
This invention relates to phosphoramidite compounds and catalyst complexes which can be used to provide enantioselective reactions including hydroamination reactions, etherification reactions and conjugate addition reactions and allylic substitution reactions, among others. In a first aspect, the present invention is directed to phosphoramidite and related compounds according to general structure (I), where Z is absent or is a group containing O, N or S, preferably O; R1 and R2 are independently an optionally substituted C1-12 alkyl group, an optionally substituted (CH2)n-aromatic group or (CH2)n-heteroaromatic group, or are linked together to form an optionally substituted aliphatic or (CH2)n-aromatic dianion of a diol, diamine, dithiol, aminoalcohol, aminohiolate or a alcoholthiol group; R3′ and R3 are each independently H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic group with the proviso that R3′ and R3 are not both H, or together R3′ and R3 form an optionally substituted C5-C15 saturated or unsaturated carbocyclic ring; R4 is H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic group; R5 is absent, H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic or (CH2)n-heteroaromatic group; Ra and Ra′ are each independently H or a C1-C3 alkyl group, or Ra and Ra′ together with the carbon to which they are attached form a optionally substituted C5-C15 saturated or unsaturated carbocyclic or heterocyclic ring, or an aromatic or heteroaromatic ring; R6 and R7 are each independently H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic group, with the proviso that R5, R6 and R7 cannot simultaneously be H, and when Ra and Ra′, together with the carbon to which they are attached, form a carbocyclic ring, heterocyclic ring or an aromatic or heteroaromatic ring, R5 is absent or is preferably H; R6 and R7 are preferably H or CH3; and each n is independently 0, 1, 2, 3, 4, 5 or 6 and wherein at least one of the carbon atoms attached to the nitrogen of the phosphoramidite group is a chiral center.
Owner:YALE UNIV
Acetal compounds and their preparation, polymers, resist compositions and patterning process
ActiveUS20100136485A1High resolutionUseful in precise micropatterningOrganic compound preparationPhotosensitive materialsResistAliphatic hydrocarbon
An acetal compound of formula (1) is provided wherein R1 is H, methyl or trifluoromethyl, R2 is a monovalent C1-C10 hydrocarbon group, R3 and R4 are H or a monovalent C1-C10 hydrocarbon group, R2 and R3 may together form an aliphatic hydrocarbon ring, and X1 is a single bond or a divalent C1-C4 hydrocarbon group. A polymer comprising recurring units derived from the acetal compound is used as a base resin to formulate a resist composition which exhibits a high resolution when processed by micropatterning technology, especially ArF lithography.
Owner:SHIN ETSU CHEM IND CO LTD
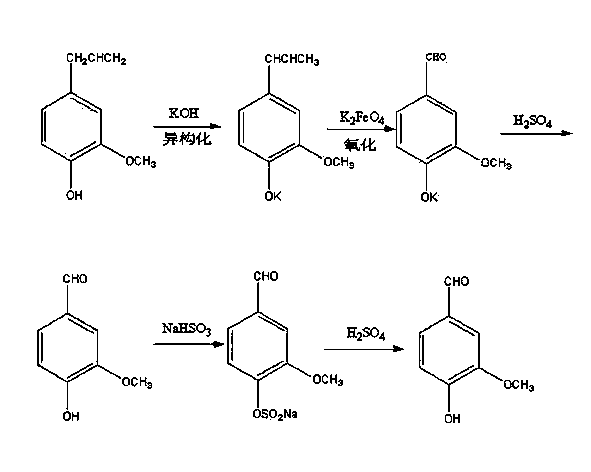
Preparation method of anisic aldehyde
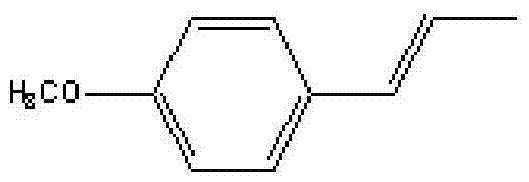
ActiveCN103497094AHigh content of purificationEasy to purifyCarbonyl compound preparation by oxidationEther preparation by isomerisationForeign matterIsomerization
The invention discloses a preparation method of anisic aldehyde and aims to provide a novel preparation method of anisic aldehyde in order to reduce the production cost of the anisic aldehyde and the environment pollution. The method comprises the following steps: isomerizing; carrying out refrigerated centrifugation; oxidizing; centrifuging; washing; and neutralizing. Estragole is subjected to steps such as isomerization, centrifugation and oxidization in the presence of a catalyst so that electrons in estragole molecules are rearranged, and foreign matters generate required anethole. The anethole is subjected to oxidization reaction under an acidic condition of manganese dioxide and transformed from ketone to aldehyde so that anisic aldehyde is obtained finally. According to the invention, the estragole is used as the raw material, the purification content of the estragole for preparing the anisic aldehyde can be up to over 97% and is about 13% higher than that of the traditional process. The preparation method is capable of remarkably increasing the yield of the anisic aldehyde and meeting the requirement for large-scale industrial production, easier to purify as well as little in equipment corrosion and environment pollution.
Owner:ZITONG HUIQUAN PERFUME CHEM
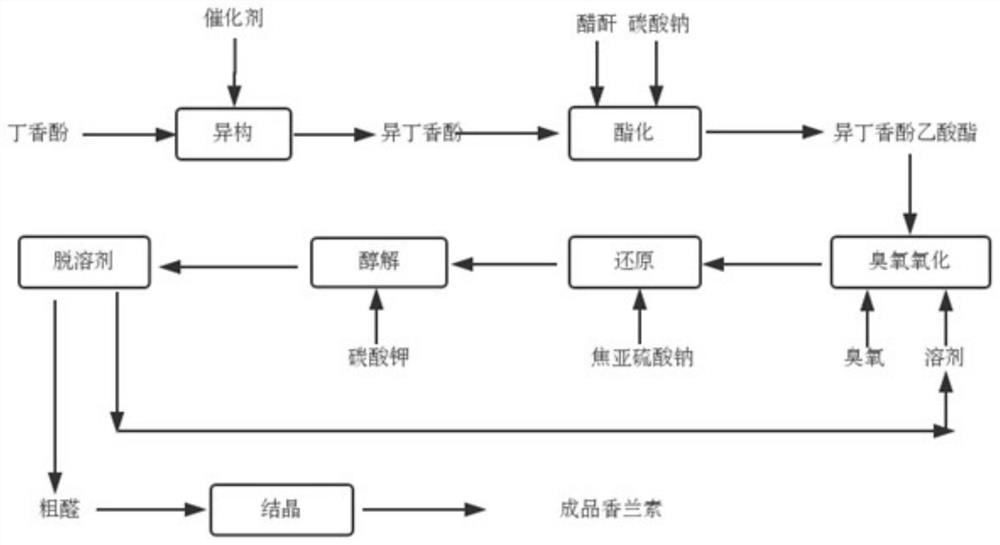
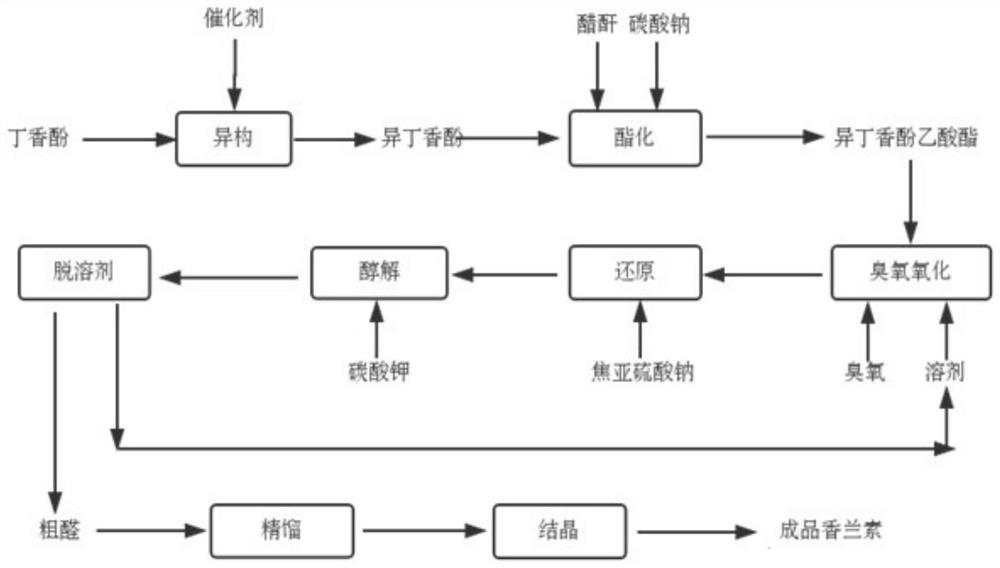
Method for preparing natural vanillin by utilizing eugenol
InactiveCN103641698ASmooth responseStable reaction conditionsOrganic compound preparationCarbonyl compound preparation by oxidationFlavorIsoeugenol
The invention discloses a method for preparing natural vanillin by utilizing eugenol. The method comprises the following steps of preparing isoeugenol sylvine, preparing an intermediate product vanillin sylvine, and preparing and purifying the target product vanillin. The reaction is stable, the condition is more moderate compared with the traditional process, the yield is higher, the product quality is better, edible spice is widely used for the strong milky fragrance of the target product, the mass production is easy to realize, and the pollution is little in the production process.
Owner:EAST CHINA UNIV OF TECH +1
Preparation method for synthesizing vanillin by using natural eugenol as raw material
InactiveCN103626643AIncrease aromaHigh yieldCarbonyl compound preparation by oxidationEther preparation by isomerisationIsomerizationIsoeugenol
The invention discloses a preparation method for synthesizing vanillin by using natural eugenol as raw material. The preparation method comprises the following steps: adding potassium hydroxide and n-amyl alcohol into a three-neck round bottom flask with volume of 250 ml and equipped with a backflow condensation tube, a magnetic stirring device and a constant-pressure dropping funnel through isomerization, dropwise adding the natural eugenol after stirring for dissolution, heating for backflow, then transferring a reaction solution into water for dilution, transferring lower-layer liquid to a separatory funnel in several times, and washing with a NaOH solution until the color of upper-layer liquid does not become light; combining all alkali liquors, and extracting with acetone, acidating and evaporating rotatably to obtain isoeugenol; adding a certain amount of nitrobenzene into the isoeugeno to oxidize double bond of propenyl of the isoeugeno, and then purifying a vanillin crude product, wherein the vanillin productivity can reach above 95.0%. The method has the advantages that the adoption of highly-toxic catalysts of carbonyl iron and the like in the isomerization process is avoided, the after-treatment technology is relatively simple, and the operation is convenient. An extraction agent can be recycled to lower the cost.
Owner:NANCHANG HANGKONG UNIVERSITY +1
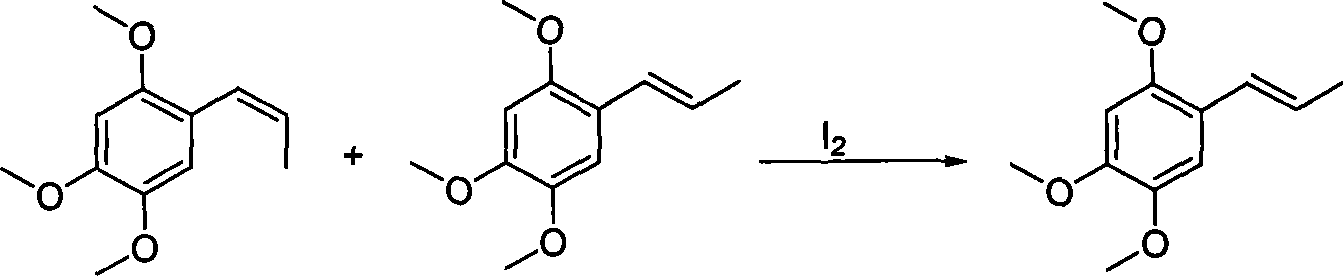
Method for synthesizing alpha-asarone
InactiveCN101215226AShort synthetic routeMild responseEther preparation by isomerisationBeta-asaroneWittig reaction
The invention discloses a method for synthesizing alpha-asarone, including the alpha / beta-asarone mixture obtained from the wittig reaction in the existing technique, then employing the alpha / beta-asarone mixture as raw materials, according to the ratio of raw materials : solvent=1 : 1-100 to dissolve the alpha / beta-asarone mixture, adding 0.025mol%-5mol% iodine catalyst into the solution and changing cis-form asarone into anti-form asarone by stirring with the solvent reflux temperature of 0-120 DEG C. The invention can control the content of cis-form asarone which has toxic side-effect, thereby obtaining acceptable product. The invention has the advantages of short synthesizing line, mild reaction, simple and easy operation, which greatly reduces cost and is adapted for mess product in industry.
Owner:HUNAN NORMAL UNIVERSITY
Method for the conversion of a Z-isomer into E-isomer
InactiveUS6844471B2Simple processOrganic compound preparationOrganic chemistry methodsArylPhotochemistry
A method of converting (Z)-1-(3,5-disubstituted phenyl)-2-(4-substituted phenyl)ethene of general formula (II) to (E)-1-(3,5-disubstituted phenyl)-2-(4-substituted phenyl)ethene of general formula (I) wherein R1, R2, and R3 are the same or different and independently represent (C1-C4)alkyl, (C1-C4)alkoxy(C1-C4)alkyl, (C1-C4)alkoxy(C1-C4)alkoxy(C1-C4)alkyl, allyl, vinyl, silyl, formyl, acyl, aryl(C1-C4)alkyl or substituted aryl(C1-C4)alkyl group. The present invention also provides a process for the conversion of (E)-(3,5-disubstituted phenyl-2-(4-substituted phenyl)ethene of general formula (I) to E-resveratrol of formula (III).
Owner:ORCHID CHEM & PHARM LTD
Method for synthesizing anethole by taking estragole oil as raw material
InactiveCN103755533ARealize reuse valueSave raw materialsEther preparation by isomerisationChemistryBy-product
The invention relates to a method for synthesizing anethole by taking estragole oil as a raw material. The estragole oil is subjected to isomerization reaction in the presence of a basic catalyst, and is frozen, crystallized and separated, so as to obtain trans-anethole. The method is cheap in raw material and is simple and safe; meanwhile, the reuse value of a by-product is achieved, and the purity of the obtained anethole can be up to over 96% by optimization on rectification and extraction conditions, so that the method is applicable to industrial production.
Owner:BEIJING FORESTRY UNIVERSITY
Preparation method of 1,2,3-trimethoxy-5-allylbenzene
InactiveCN104230681AHigh yieldHigh purityOrganic compound preparationEther preparation by ester reactionsBiochemical engineeringCombinatorial chemistry
The invention provides a preparation method of 1,2,3-trimethoxy-5-allylbenzene. The synthetic route of the preparation method is as shown in the specification. According to the preparation method disclosed by the invention, the synthetic process conditions are simple, the cost is low, the total yield of the reaction can be above 49% and the preparation method is suitable for industrialized mass production and has significant business values.
Owner:程文峰
Process for isomerizing and converting (Z)-olefins to (E)-olefins
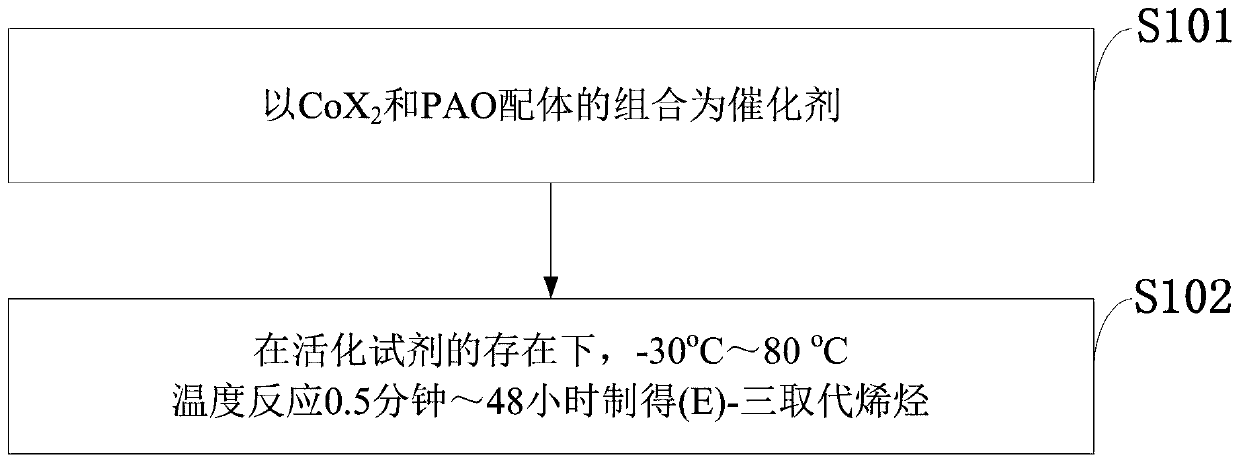
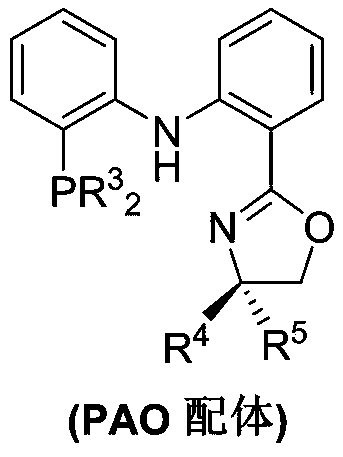
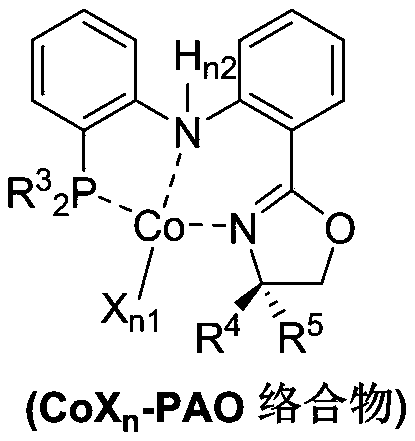
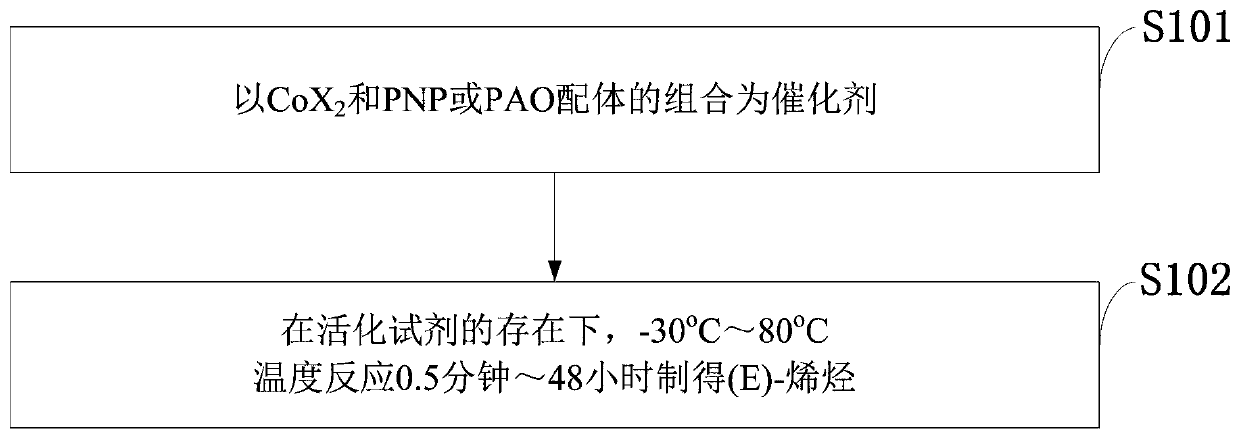
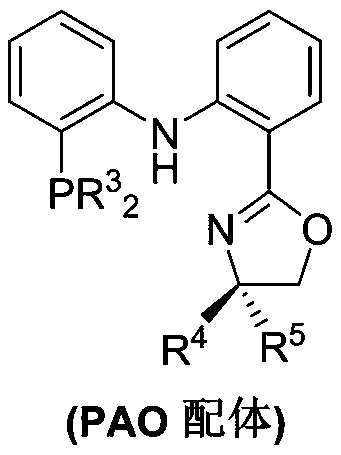
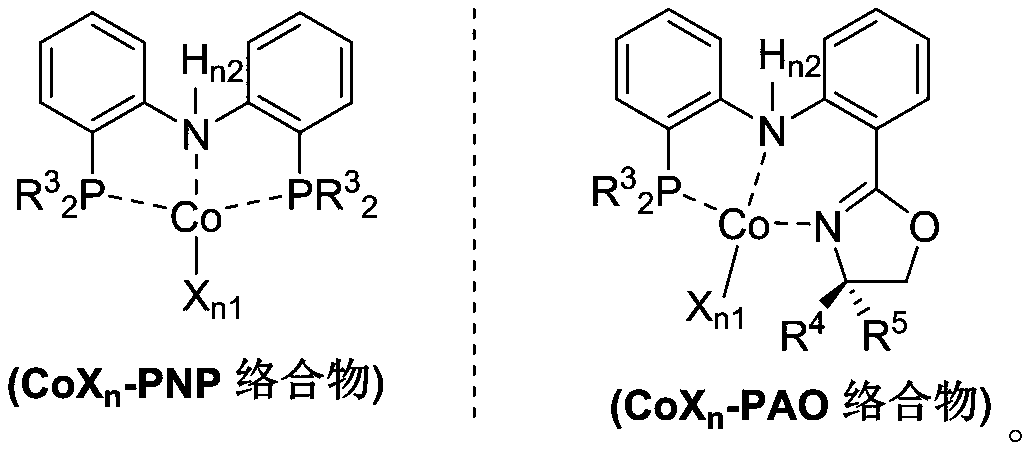
PendingCN110878001ARealize isomerization conversionImprove efficiencySilicon organic compoundsHydrocarbon by isomerisationIsomerizationPtru catalyst
The invention belongs to the technical field of metal catalytic synthesis, and discloses a method for isomerizing and converting (Z)-olefins into (E)-olefins. The (E)-olefins are prepared through a reaction at -30-80 DEG C for 0.5-48 h by using a combination of CoX2 and a PNP or PAO ligand as a catalyst in the presence of an activating reagent; and a molar ratio of the (Z)-olefins to the CoX2 to the PNP or PAO ligand to the activating reagent is 1:(0.00001-0.10):(0.00001-0.10):(0.00003-0.30). The catalyst used in the invention is the combination of the cheap metal cobalt salt and the simple and easily available ligand, no other toxic transition metal (such as ruthenium, rhodium and palladium) salt is added in the reaction, and the method also has the advantages of cheap and easily available raw material, good functional group tolerance, mild reaction conditions, simplicity in operation, and e atom economy of 100%.
Owner:WENZHOU UNIVERSITY
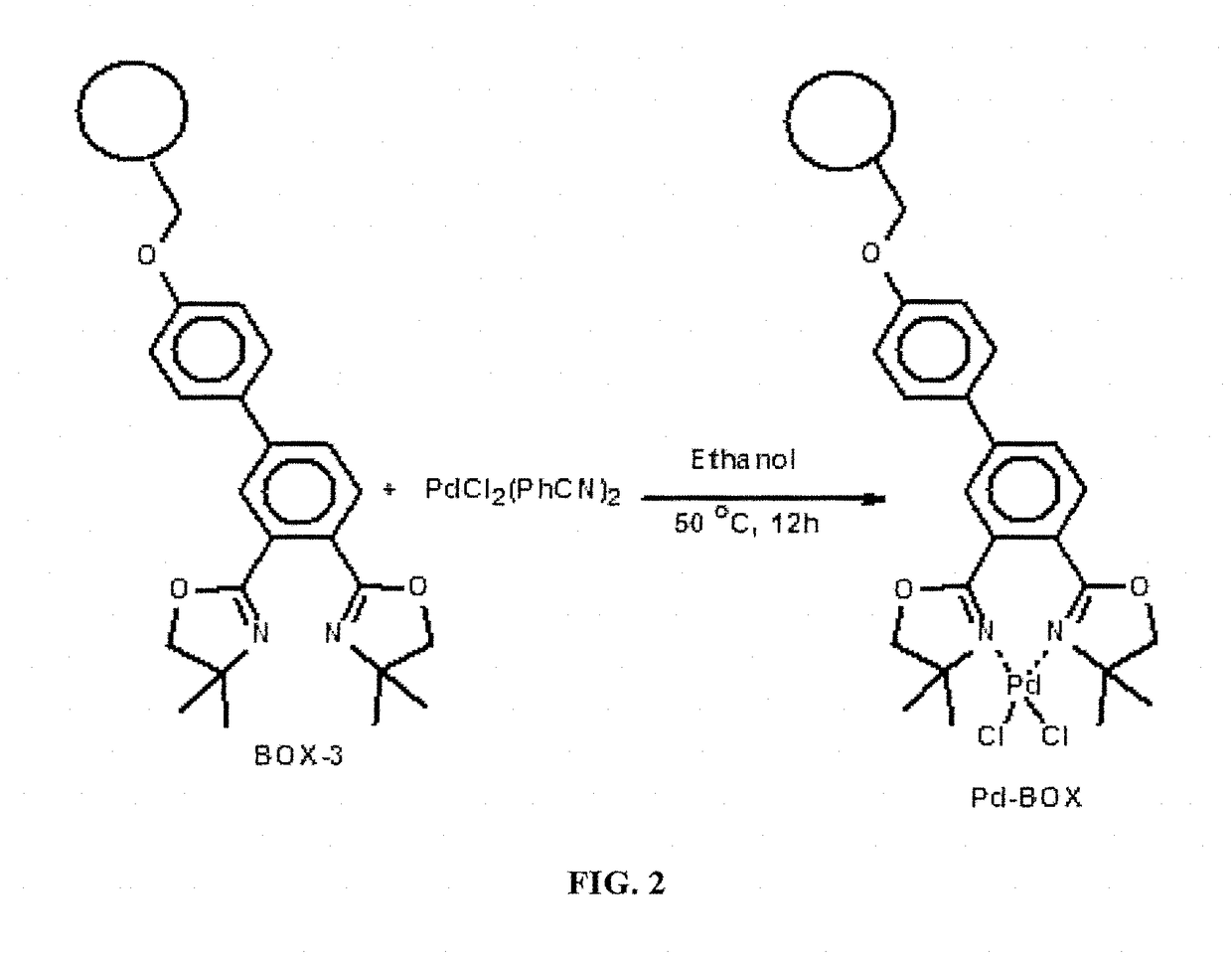
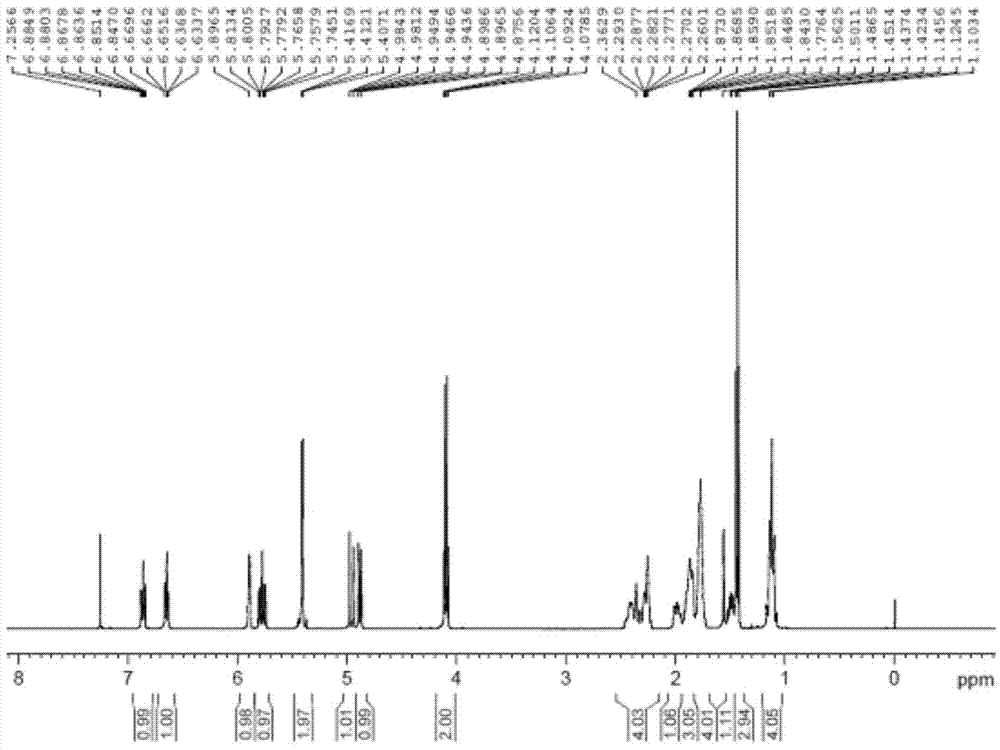
Solid-supported palladium (II) complex as a heterogeneous catalyst for cross coupling reactions and methods thereof
InactiveUS20170275318A1Carboxylic acid nitrile preparationAmino preparation from aminesChemical transformationCoupling
A solid-supported catalyst ligand which chelates palladium (II) species to form a complex that functions as a heterogeneous catalyst that is stable and can be recycled without significantly losing any catalytic activity in a variety of chemical transformations, a method for producing the solid-supported catalyst ligand and a method for catalyzing a palladium cross-coupling reaction, such as the Suzuki-Miyaura, Mizoroki-Heck, and Sonagashira reactions.
Owner:KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS
Preparation method of trienes liquid crystal monomers
ActiveCN104844428AOvercome the shortcoming of extremely low yieldFew reaction stepsLiquid crystal compositionsHalogenated hydrocarbon preparationIsomerizationCyclohexenone
The invention relates to a preparation method of trienes liquid crystal monomers. The method comprises the following steps: using alkyl 2,3-difluorobenzene, alkoxy 2,3-difluorobenzene, 1-bromine-4-alkyl 2,3-difluorobenzene or 1-bromine-4-alkoxy 2, 3-difluorobenzene as raw materials, performing a metallization reaction or a Grignard reaction and a reaction with a cyclohexanone compound, performing hydroxyl protection, a Wittig reaction, alkene-ether hydrolysis, hydroxyl deprotection and alcohol dehydration so as to obtain an intermediate of which the formula is shown in the specification, performing the Wittig reaction once again, and performing a double-bond isomerization reaction so as to obtain the finished product, wherein three steps of the reactions of the alkene-ether hydrolysis, the hydroxyl deprotection and the alcohol dehydration can be combined to be performed in one step. The preparation method disclosed by the invention overcomes the disadvantage that the yield of cyclohexenone in the Wittig reaction is extremely low in the conventional preparation method of the compound, the yield of products is increased, the production cost is reduced, the large-scale production is facilitated, and the preparation method has very good application prospects.
Owner:VALIANT CO LTD
Method for preparing halogenated benzo [alfa] fluorenol
InactiveCN102659512AHigh yieldFunction increaseOrganic compound preparationPreparation by halogen introductionReaction temperatureSide reaction
The invention provides a method for preparing halogenated benzo [alfa] fluorenol. The method comprises the following step of performing series electrophilic cyclization reaction on 3-aryl-1-(2-(2-aryl ethinyl) phenyl) propargyl-2-alcohol serving as a reaction substrate and various electrophilic reagents such as halogenated succinimide (NXS, X=I, Br and Cl) or simple substance iodine (I2), simple substance bromine (Br2) or iodine chloride (ICl) at the temperature of 0 and 15 DEG C for 10 and 15 hours under the catalysis of AgOTf, and is a 'one-pot method' for efficiently preparing the Halogenated benzo [alfa] fluorenol. The method has the advantages of mild reaction conditions, low cost, less side reaction, high product purity, is easy to operate and can be applied to mass production of the halogenated benzo [alfa] fluorenol; and moreover, separation and purification can be conveniently realized.
Owner:JIANGXI NORMAL UNIV
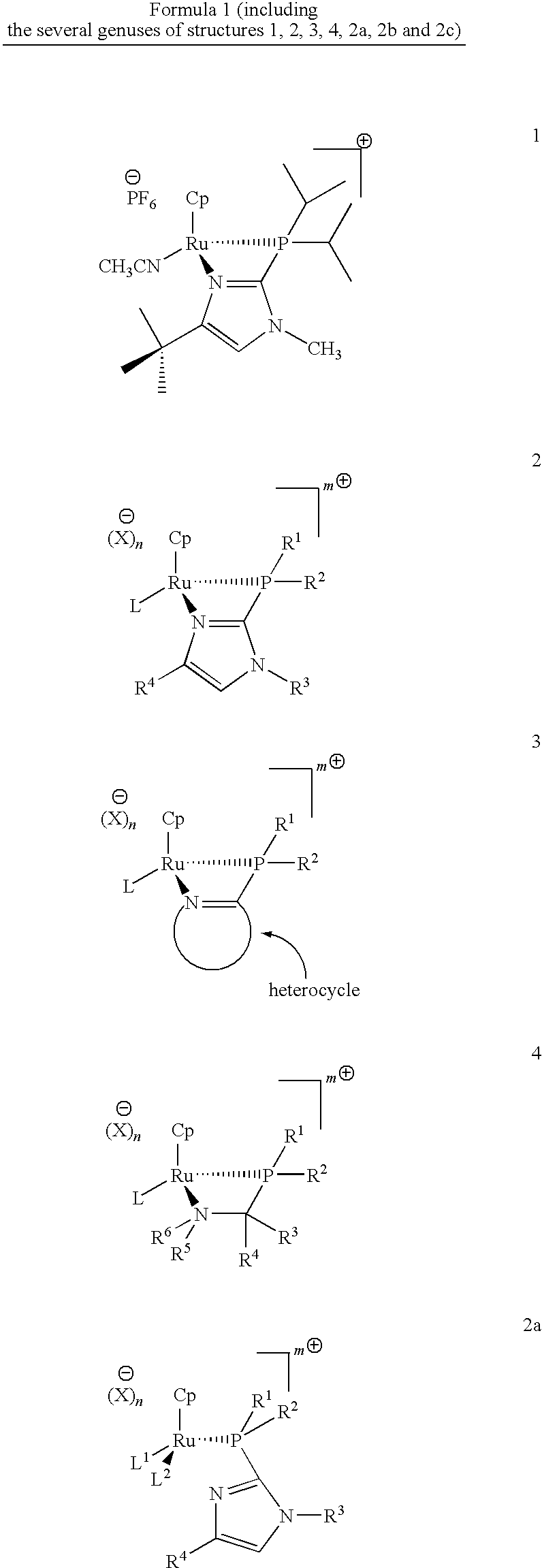
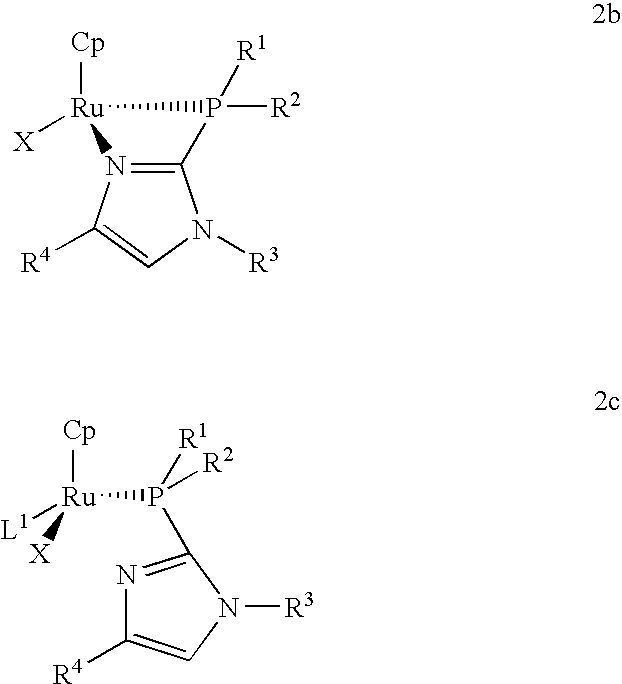
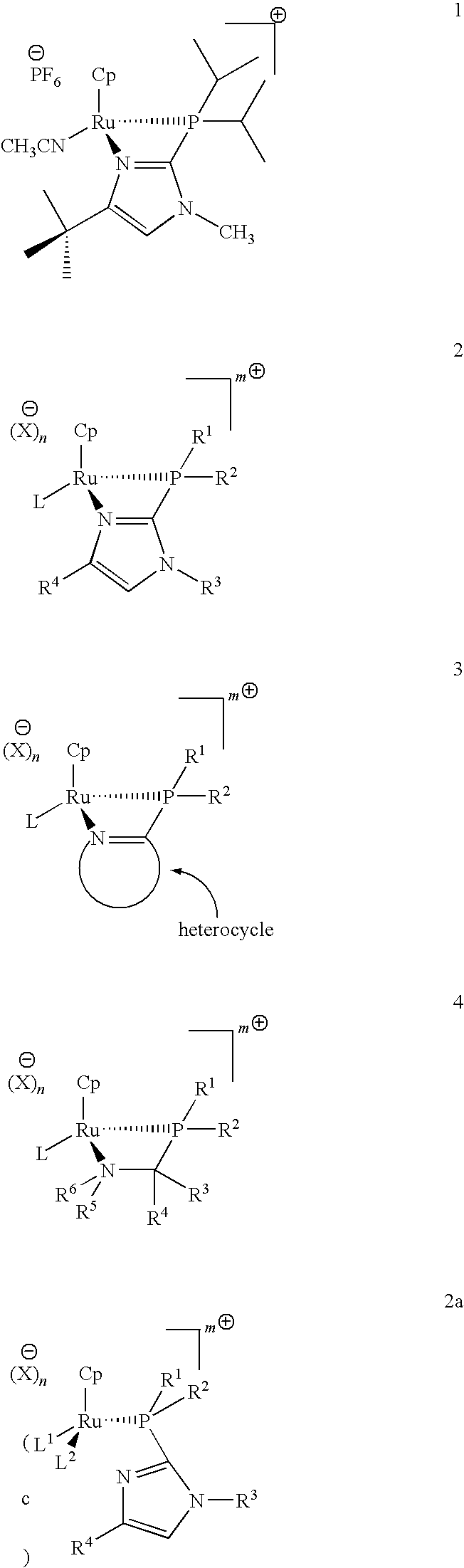
Catalysts for alkene isomerization and conjugating double bonds in polyunsaturated fats and oils
The invention provides ruthenium-comprising catalysts, and methods of making and using them, for conjugating double bonds in polyunsaturated hydrocarbons, including polyunsaturated fatty acid derivatives, such as natural fats and oils which comprise (contain) more than one carbon to carbon double bond—where the double bonds are separated by, e.g., a methylene, ethylene or propylene or longer group. The invention provides compositions and methods for treating fats and oils comprising “interrupted” (e.g., “methylene-, ethylene- or propylene-interrupted”) double bonds to generate isomers with “conjugated” double bonds. The invention also provides compositions, and methods of making and using them, for making catalysts on a solid support. In one aspect, these catalysts are for alkene isomerization or exchange of alkene hydrogens for other isotopes. The invention provides heterocyclic resin-based compositions, and methods of using them, for making catalysts for alkene isomerization and exchange of hydrogens for deuterium or tritium isotopes.
Owner:SAN DIEGO STATE UNIVERSITY
Method for converting estragole into trans-anethole
InactiveCN104311399AReduce dosageShort reaction timeEther separation/purificationOrganic chemistry methodsIsomerismsSide reaction
The invention relates to a method for converting estragole into trans-anethole. The method comprises the steps of carrying out isomerism reaction on the estragole and NaOH solution under pressurization so as to produce the trans-anethole, then carrying out acid pickling and neutralizing on the trans-anethole, washing, carrying out freeze crystalizing, centrifuging to separate, and vacuum rectifying to obtain the high purity trans-anethole with the purity of greater than 99.5%. The method has short reaction time, uses little alkaline, avoids contacting with the air, reduces side reactions like oxidative polymerization, improves the yield of the trans-anethole.
Owner:GUANGXI FORESTRY RES INST
Method for landfill of gas fluidification dimethyl ether fuel by garbage
ActiveCN101130487AShort processAvoid separationHydrogen productionEther preparation by isomerisationCatalytic reformingThermal energy
The invention discloses a preparing method of dimethyl ether, which comprises the following steps: choosing garbage embedding gas as initial raw material; choosing methane and CO2 as main chemical element; setting near volume fraction; proceeding catalytic reforming reaction; generating synthetic gas with hydrogen-carbon ratio near 1; setting the upstream synthetic gas enriched with CO as raw material; synthesizing dimethyl ether in slurry state bed reactor; using the garbage embedding gas as main energy; providing heat energy for system; using the rear gas as fuel; providing heat for the system; adopting deep cooled method; condensing the dimethyl ether to liquid; separating from the synthetic gas; getting the cooled energy from absorption refrigerating machine group. This invention provides a new approach for garbage treating site to utilize embedding gas, which can transferring the waste source to clean energy.
Owner:中科合肥煤气化技术有限公司
Isoeugenol synthetizing method
ActiveCN103408407AImprove solubilityRaise the ratioEther preparation by isomerisationRotary evaporatorIsoeugenol
Owner:CHONGQING THRIVE CHEM
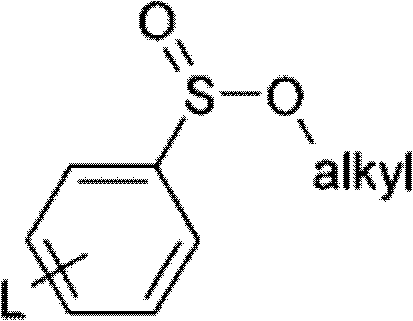
Method for manufacturing olefinic derivative composition
ActiveCN105732334ARaise the ratioHydrocarbon by isomerisationOrganic compound preparationChemical synthesisLiquid crystal
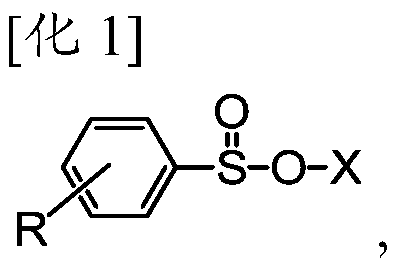
The invention provides a method for manufacturing olefinic derivative composition. The invention provides a method for manufacturing a compound. The compound is useful, has an E-alkene structure and serves as an intermediate or a prototype for the manufacturing of chemical synthetics such as medicine, pesticides, liquid crystals. Through arylsulfinate acting on a composition containing E-alkene derivative and Z-alkene derivative while heating is performed, the alkene derivative composition having more than 80 % of the E-alkene derivative is manufactured. The composition thus obtained has a higher ratio of the E-alkene derivative with respect to the Z-alkene derivative (E body / Z body), so that an object E-alkene derivative can be obtained in a higher ratio than that of traditional methods.
Owner:DIC CORP
Method for olefin isomerization
ActiveCN102731237ALiquid crystal compositionsHydrocarbon by isomerisationPolymer scienceIsomerization
The invention relates to a method for preparing an olefin composition of E-construction, comprising isomerization steps for transforming an olefin composition of Z-construction into the olefin composition of E-construction under the isomerization situation, wherein the olefin composition of E-construction is in solid phase.
Owner:MERCK PATENT GMBH
Synthesis method of isoeugenol
InactiveCN103848728AEasy to separateIncrease relative volatilityEther separation/purificationEther preparation by isomerisationUltrasound - actionDistillation
The invention provides a synthesis method of isoeugenol. The method comprises the following steps: adding ground cloves powder to a potassium hydroxide solution which is 1mol / L in concentration and ethanol, and keeping under an ultrasonic condition at 100-130 DEG C for 1-2 hours to obtain a decomposition solution; stirring and mixing the decomposition solution with methylbenzene, heating and distilling under a vacuum state, controlling distilling and heating temperature at 50-90 DEG C, controlling temperature of a distillation gas volatilization outlet at 35-45 DEG C, and condensing and collecting distillation gas through a condenser to obtain low boiling point impurities; continuing to distill rest filtrate after removing the low boiling point impurities, controlling distilling and heating temperature at 100-120 DEG C, controlling temperature of the distillation gas volatilization outlet at 90-105 DEG C, and condensing and collecting the distillation gas through the condenser to obtain the isoeugenol. The synthesis method disclosed by the invention is light in environmental pollution, more simple to operate and quite low in production cost; moreover, content of trans-isoeugenol can achieve more than 95% in the isoeugenol.
Owner:ZIBO VOCATIONAL INST
Method for synthesizing vanillin from eugenol through ozone oxidation
PendingCN112110807AMild reaction conditionsReaction conditions are easy to controlOrganic compound preparationCarboxylic acid esters preparationChemical synthesisIsomerization
The invention relates to the technical field of organic chemical synthesis, in particular to a method for synthesizing vanillin from eugenol through ozone oxidation. The method comprises the followingsteps: adding dichlorotris(triphenylphosphine)ruthenium serving as a catalyst into eugenol to perform isomerization reaction to obtain isoeugenol, performing esterification reaction to obtain isoeugenol acetate, performing ozonation, and performing reducing; and carrying out alcoholysis and refining on the acetyl vanillin to obtain a pure product. The method solves the problems of low productionyield, use of a metal oxidant and difficult treatment of wastewater in the eugenol method in the prior art, eugenol isomerization adopts dichlorotris(triphenylphosphine)ruthenium as a catalyst, no wastewater is generated in the process, isoeugenol acetate is oxidized by ozone, the use of a heavy metal oxidant is avoided, and the method conforms to the green development trend. The method has the advantages of environmental protection and high yield.
Owner:成都三香汇香料有限公司
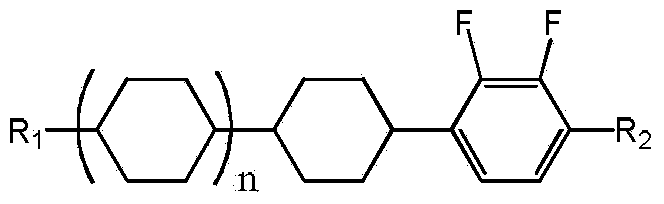
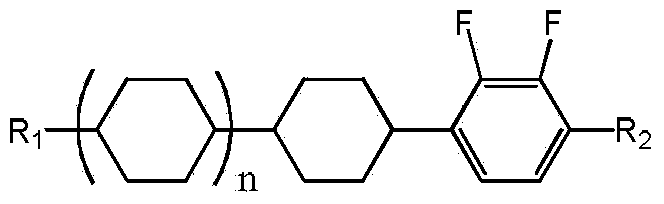
Conversion method of 1-cyclohexyl-2,3-difluorobenzene derivative cis-trans-isomers
ActiveCN103709017AIncrease productionLow costOrganic chemistry methodsHalogenated hydrocarbon preparationHydrogen atmosphereLiquid crystal
The invention discloses a conversion method of 1-cyclohexyl-2,3-difluorobenzene derivative cis-trans-isomers. The method comprises the following steps: in a hydrogen atmosphere, evenly mixing 1-cyclohexyl-2,3-difluorobenzene derivative cis-trans-isomers disclosed as Formula I and a catalyst to perform catalytic reaction, and after the reaction finishes, obtaining the 1-cyclohexyl-2,3-difluorobenzene derivative trans-isomers disclosed as Formula I. The method can convert most cis-structure products into a trans-structure liquid crystal material, avoids the direct discharge of the cis-structure products as waste compounds, reduces the pollution, and greatly increases the yield of the trans-structure liquid crystal material, thereby lowering the production cost of the 1-cyclohexyl-2,3-difluorobenzene derivative liquid crystal monomer. Formula I.
Owner:SHIJIAZHUANG CHENGZHI YONGHUA DISPLAY MATERIALS CO LTD
Ruthenium olefin metathesis catalyst with adjacent space steric structure as well as preparation method and application thereof
ActiveCN109225334ASimple stepsMild reaction conditionsRuthenium organic compoundsOrganic compound preparationEthylenediamineRoom temperature
The invention discloses a ruthenium olefin metathesis catalyst with an adjacent space steric structure as well as a preparation method and application thereof. The catalyst has the following general formula and the preparation method comprises the following steps: (1) Zn (OAc) 2.2H2O and a compound (I) are dissolved in an isopropanol solvent, ethylenediamine is added for reaction, and precipitatedsolids are filtered after the reaction and washed with methanol and chloroform, thereby obtaining a compound (II) after vacuum drying; and (2) Grubbs-Hoveyda II and the compound (II) are added into adried tetrahydrofuran solvent under nitrogen condition for reaction, the solvent is centrifuged under N2 after the reaction and then collected and dried until only solids are left, and the solids arewashed by anhydrous ether, thereby obtaining the compound (III), namely the ruthenium olefin metathesis catalyst provided by the invention. The method provided by the invention is simple in steps andmild in reaction condition, and the cis-structural product with specific configuration can be obtained by reaction under room temperature, therefore the invention has a broad application prospect.
Owner:JILIN INST OF CHEM TECH
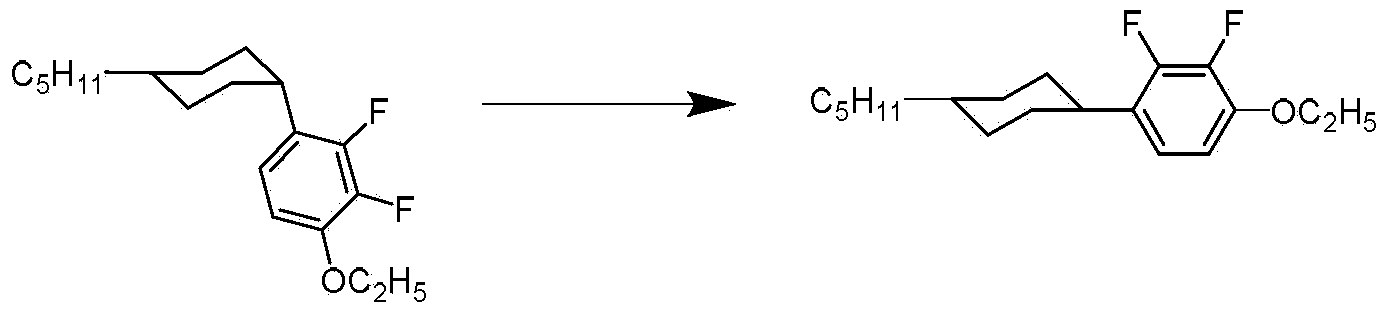
Method for stereoselective synthesis of (E)-trisubstituted olefin
ActiveCN110903172AHigh stereoselectivityImprove efficiencyGroup 4/14 element organic compoundsHydrocarbon by isomerisationChemical industryPtru catalyst
Belonging to the technical field of metal catalytic synthesis, the invention discloses a method for stereoselective synthesis of (E)-trisubstituted olefin. The method includes: taking 1, 1-disubstituted olefin as the raw material, and adopting a combination of CoX2 and PAO ligand as the catalyst; and in the presence of an activating reagent, carrying out reaction for 0.5min-48h at a temperature ranging from -30DEG C to 80DEG C to prepare (E)-trisubstituted olefin. Compared with the prior art, the method has the advantages of more economical, efficient and environment-friendly catalyst, good tolerance of the reaction functional group, mild reaction conditions, simple operation, no need for participation of additional reagents, and atom economy of 100%. In addition, the reaction has no needof any other toxic transition metal (like ruthenium, rhodium, palladium, etc.) salt, therefore the method has great practical application value in pharmaceutical and food chemical industry.
Owner:WENZHOU UNIVERSITY
Electrically catalyzed process of preparing green fuel dimethyl ether with coarse ethanol product in hydrated proton reactor
InactiveCN101020623AMaintain stabilityAlleviate \"Sannong\"Ether preparation by isomerisationHydration reactionProton
The process of preparing green fuel dimethyl ether with coarse ethanol product in an electrically catalyzed hydrated protonic reactor includes re-arranging ethanol molecule under the actions of electrode and double function metal-acid catalyst to obtain dimethyl ether product, and collecting dimethyl ether. The present invention uses material from biomass and has no fossil resource.
Owner:BEIJING GUOLIYUAN POLYMER SCI & TECH R & D CENT
Resveratrol synthesis preparation method
InactiveCN104326880AMild reaction conditionsHigh yieldOrganic compound preparationGroup 5/15 element organic compoundsXylyleneFood additive
The invention relates to a resveratrol preparation method which belongs to the field of food additives and preparing methods thereof. The resveratrol synthesis preparation method comprises the following steps: (1) using 4-methoxybenzyl bromide, triphenyl phosphine and xylene as raw materials for synthesis of triphenyl 4-methoxybenzyl phosphorus bromide (compound I); (2) using the compound I, tetrahydrofuran and 3, 5-dimethoxy benzaldehyde as raw materials for synthesis of a cis / trans-3, 4, 5-trimethoxy-1, 2-diphenylethene mixture (compounds II); (3) using the compound II, tetrahydrofuran and diphenyl disulfide as raw materials for synthesis of trans-3, 4, 5-trimethoxy-1, 2-diphenylethene (compound III); and (4) using the compound III, boron tribromide and methylene chloride as raw materials for synthesis of trans-3, 4, 5-trihydroxy-1, 2-diphenylethene (compound IV), namely resveratrol. The resveratrol synthesis preparation method has the advantages of mild reaction conditions, high yield (on the basis of the 4-methoxybenzyl bromide, the total yield is 66%), high purity (more than 98%) and the like.
Owner:西安莹朴生物科技股份有限公司
Preparation method for beach-chair-typed nonacene compounds
ActiveCN108129276AImprove stabilityImprove the deficiency of being easily oxidized and deterioratedOrganic compound preparationOrganic chemistry methodsBenzeneOrganic solar cell
The present invention relates to a preparation method for beach-chair-typed nonacene compounds. The method comprises the following steps: under the protection of an inert gas, 6-(bis(4-(dodecoxy)phenyl)methylene)-13-(bromo(phenyl)methylene)-6,13-dihydropentacene and 1,4-benzenediboronic acid pinacol diester are dissolved into an appropriate solvent, under the action of a catalyst, through a coupling reaction, 1,4-di((13-(bis(4-(dodecyl)phenyl)methylene)dihydropentacene-6(13H)-phenylmethylene)benzene is obtained, then under the action of an iodine element, irradiation is performed by using an ultraviolet mercury lamp, and through a photocyclization reaction, the final product c-HBC dimers, namely, the beach-chair-typed nonacene derivatives are obtained. According to the method provided by the invention, the excellent-performance stable beach-chair-typed nonacene derivatives are synthesized for the first time, and the compounds can be used in the fields such as organic field effect transistors, organic light emitting diodes and organic solar cells.
Owner:SHANGHAI NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Method for preparing halogenated benzo [alfa] fluorenol Method for preparing halogenated benzo [alfa] fluorenol](https://images-eureka.patsnap.com/patent_img/730ecbfb-2118-4186-9b35-a5d7820d2da6/HSA00000715058900011.PNG)
![Method for preparing halogenated benzo [alfa] fluorenol Method for preparing halogenated benzo [alfa] fluorenol](https://images-eureka.patsnap.com/patent_img/730ecbfb-2118-4186-9b35-a5d7820d2da6/HSA00000715058900012.PNG)
![Method for preparing halogenated benzo [alfa] fluorenol Method for preparing halogenated benzo [alfa] fluorenol](https://images-eureka.patsnap.com/patent_img/730ecbfb-2118-4186-9b35-a5d7820d2da6/BSA00000715058800041.PNG)