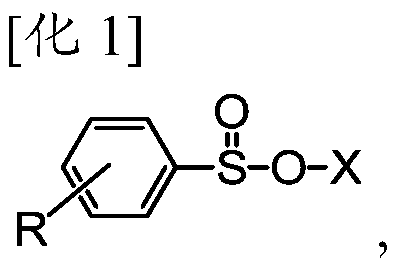
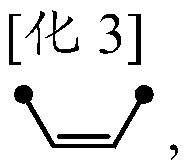
Method for manufacturing olefinic derivative composition
A manufacturing method and a derivative technology, which can be used in the preparation of organic compounds, halogenated hydrocarbons, chemical instruments and methods, etc., and can solve the problems of high cost and deterioration of yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0196] (Example 1) Production of 1-ethoxy-2,3-difluoro-4-[trans-4-((E)-1-propenyl)cyclohexylmethoxy]benzene using hydrochloric acid
[0197] [chem 37]
[0198]
[0199]E / Z olefin composition (81.5 g, E Body / Z body=6 / 94), sodium benzenesulfinate (6.5 g) and toluene (200 mL) were mixed and heated to an internal temperature of 45°C. After adding 10 mass % hydrochloric acid (28.7g) at internal temperature 45 degreeC, it heated to internal temperature 85 degreeC over 15 minutes. The reaction solution was analyzed by gas chromatography, and it was found that E body / Z body=87 / 13. After the reaction solution was cooled to room temperature, water (100 mL) was added for liquid separation, and the organic layer was washed with water (100 mL), saturated aqueous sodium carbonate solution (100 mL) and saturated brine (100 mL), and anhydrous sodium sulfate was added. to dry. Sodium sulfate was filtered off, concentrated, and purified by silica gel column chromatography to obtain 1-eth...
Embodiment 2
[0204] (Example 2) Production of 1-ethoxy-2,3-difluoro-4-[trans-4-((E)-1-propenyl)cyclohexylmethoxy]benzene using acetic acid
[0205] [chem 39]
[0206]
[0207] E / Z olefin composition (89.7 g, E Body / Z body=6 / 94), sodium benzenesulfinate (7.1 g) and toluene (225 mL) were mixed, and heated to an internal temperature of 95°C. Acetic acid (45 mL) was slowly added at an internal temperature of 95°C, followed by further stirring at 95°C for 3 hours. After cooling to room temperature, water (225 mL) was added for liquid separation, and the organic layer was washed sequentially with water (225 mL), saturated aqueous sodium carbonate solution (225 mL) and saturated brine (225 mL), and dried by adding anhydrous sodium sulfate. Sodium sulfate was filtered off, concentrated, and purified by silica gel column chromatography to obtain 1-ethoxy-2,3-difluoro-4-[trans-4-(1-propenyl)cyclohexylmethoxy] E / Z olefin composition of benzene (87.4 g, yield 97%, E body / Z body=90 / 10).
Embodiment 3
[0208] (Example 3) trans,trans-4-(4-ethoxy-2,3-difluorophenyloxymethyl)-4'-((E)-1-propenyl)bicyclohexyl ester manufacturing
[0209] [chemical 40]
[0210]
[0211] The isomerization reaction was carried out under the same conditions as in Example 2, and as a result, trans,trans-4-(4-ethoxy-2,3-difluorophenyloxymethyl)-4'-(1 - E / Z olefin composition of propenyl)bicyclohexyl ester (48.6 g, yield 98%, E body / Z body=83 / 17).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com