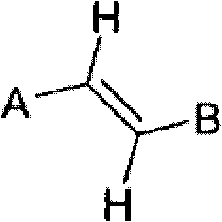
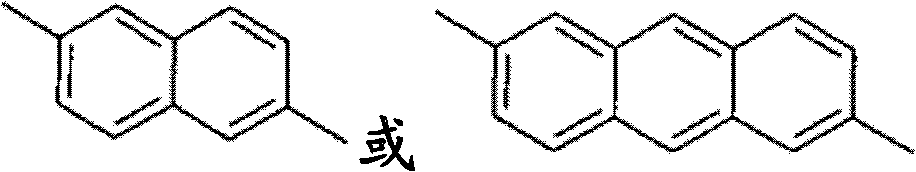
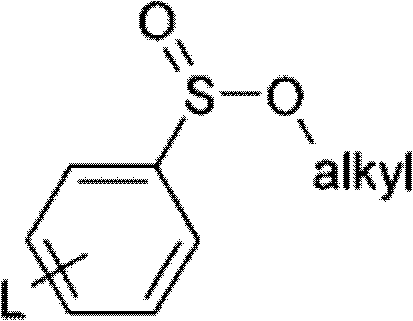
Method for olefin isomerization
A technology of olefin and configuration, applied in the field of preparation of E-configuration alkenyl compounds, can solve problems such as insufficient to avoid separation of Z isomer and other by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086]
[0087] 67 g of p-toluenesulfinic acid Na salt, 435 g of compound 1a / b (crude, from the Wittig reaction) and 784 g of n-heptane were initially introduced into a 4 L stirring apparatus. 57.0 g of hydrochloric acid (25%), 351.7 g of water and 87.1 g of ethanol were added to the suspension.
[0088] The suspension was warmed to 81 °C with stirring. A clear phase appeared at 70°C. After 3.5 h, the still-hot lower aqueous phase was separated. The organic phase was then passed through with 250 g of NaHCO 3 The solution was washed with stirring for 30 min, and the aqueous phase was separated (pH=8). The organic phase was finally washed again by shaking with 250 ml of water, and the aqueous phase was separated again (pH=7). Heptane was distilled off from the organic phase to obtain a residue (isomer distribution 85:15 E / Z, cyclohexane rings all in 1,4-trans configuration, about 1.6% of cis-configured rings present hexane ring).
[0089] The residue comprising 1a / b is ...
Embodiment 2
[0108]
[0109] Compound 2a / b having a crude content of 75:25 (E / Z) obtained by isomerization analogously to Example 1 with sulfinic acid was modified at about 25° C. in an apparatus as in Example 1 Irradiate for 27h.
[0110] The resulting isomer ratio: 94:6 (E / Z).
Embodiment 3
[0112]
[0113] The reaction was carried out analogously to Example 1.
[0114] The resulting isomer ratio: 94:6 (E / Z).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| isotropization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com