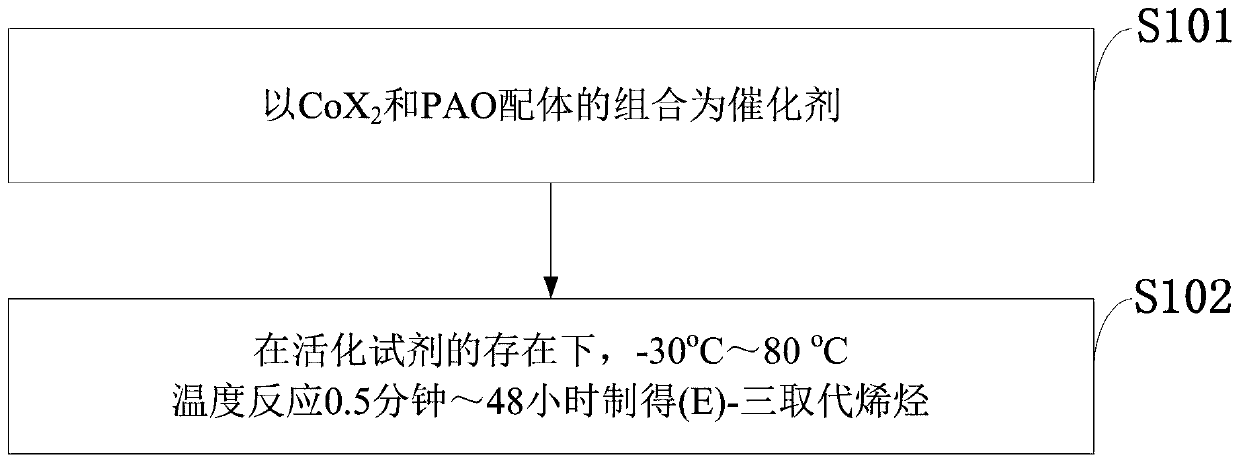
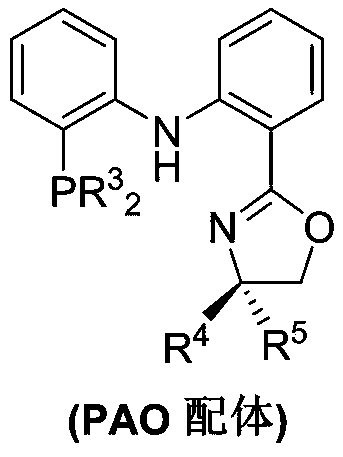
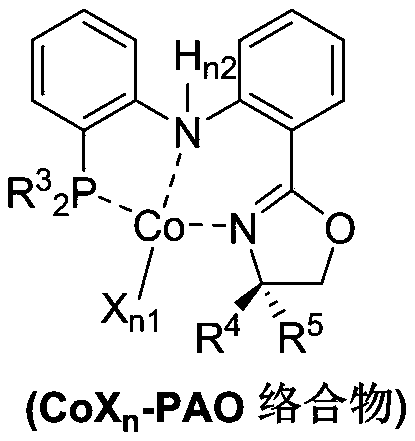
Method for stereoselective synthesis of (E)-trisubstituted olefin
A stereoselective, three-substitution technology, which is applied in the preparation of halogenated hydrocarbons, organic chemical methods, chemical instruments and methods, etc., can solve the problems of expensive catalyst, large amount of catalyst, and long reaction time, etc., and achieve high industrial application prospects, Great practical application value, high stereoselective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Synthesis of E-trisubstituted olefins by catalytic isomerization
[0044]
[0045] Reaction operation: at 25°C, add CoCl to a dry reaction test tube 2 (0.01mmol), PAO ligand (0.01mmol), alkene (10mmol), toluene (1mL), injected into triethylsodium borohydride (0.03mmol), then stirred at room temperature for 10 minutes and separated by column chromatography to obtain the product .
[0046] Product 1: (E)-1-(But-2-en-2-yl)-4-methoxybenzene
[0047]
[0048] Colorless oily liquid, yield>99%, E / Z>99 / 1. 1 H NMR: (400.0MHz, CDCl 3 )δ7.33-7.28(m,2H),6.87-6.82(m,2H),5.83-5.73(m,1H),3.80(s,3H),2.03-1.98(m,3H),1.78(dq, J=6.8,1.2Hz,3H).
[0049] Product 2: (E)-1-(But-2-en-2-yl)-3-methoxybenzene.
[0050]
[0051] Colorless oily liquid, yield>99%, E / Z>99 / 1. 1 H NMR: (400.0MHz, CDCl 3 )δ7.22(t, J=7.8Hz, 1H), 6.97(d, J=7.8Hz, 1H), 6.93-6.89(m, 1H), 6.79-6.74(m, 1H), 5.91-5.83(m ,1H),3.81(s,3H),2.01(s,3H),1.79(dq,J=6.8,1.0Hz,3H).13C NMR:(100.6MHz,CDCl3)d...
Embodiment 2
[0115] Embodiment 2: the asymmetric epoxidation reaction comparison of three substituted alkenes
[0116]
[0117] Reaction operation: at room temperature, in a dry reaction test tube, add Salen-Mn catalyst (0.05mmol), olefin (1mmol), dichloromethane (DCM) (2mL), sodium hypochlorite (NaOCl) (1.5mmol), then After stirring for 3 hours at °C, the product was isolated by column chromatography.
[0118] The reaction using the E / Z-trisubstituted olefin mixture (E / Z=20 / 80) as the raw material gives the mixed product of the epoxidation reaction in a ratio of 1:4. The reaction starting from E-trisubstituted alkenes (E / Z>99 / 1) gave product 24.
[0119] Product 24: (2S,3S)-2,3-dimethyl-2-phenyloxirane
[0120]
[0121] Colorless oily liquid, yield 82%, 93% ee. 1 HNMR: (400.0MHz, CDCl 3 )δ7.20-7.37 (m, 5H), 2.97 (q, J = 5.6Hz, 1H), 1.69 (s, 3H), 1.45 (d, J = 5.4Hz, 3H); 13 C NMR (100MHz, CDCl 3 )δ: 143.5, 128.7, 127.6, 125.4, 62.9, 60.6, 17.8, 14.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com