Isoeugenol synthetizing method
A synthetic method, the technology of isoeugenol, which is applied in the field of isoeugenol synthesis, can solve the problems of reducing the proportion of cis structure, increasing the production cost, and failing to satisfy the product, so as to simplify the production process, avoid environmental pollution, and improve the trans The effect of product ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
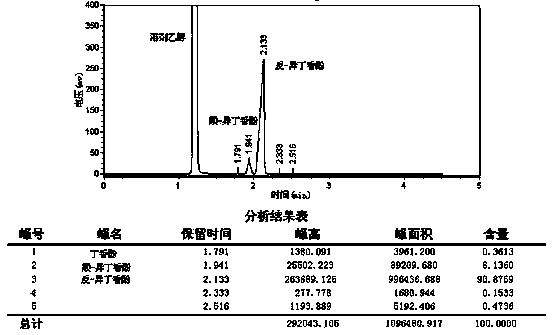
Examples
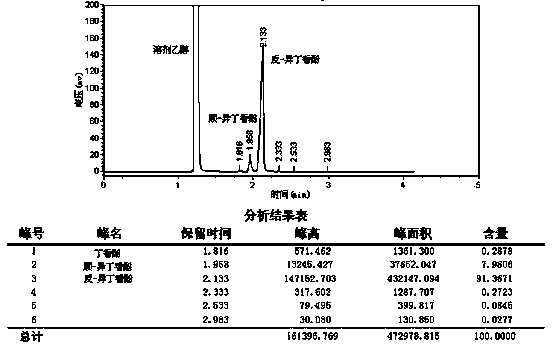
Embodiment 1
[0040] A synthetic method for isoeugenol, comprising the following steps:
[0041] a. Add 110.0 grams of 1,2-propanediol and 35.0 grams of potassium hydroxide in sequence in the three-necked flask, stir and mix thoroughly, add 30.0 grams of eugenol under nitrogen protection, and continue to stir and mix;
[0042] b. Rapidly raise the temperature of the reactant to 165°C within 20 minutes under the protection of nitrogen, and reflux for 8 hours. This reaction is a closed reaction;
[0043] c. Cool down to 50-60°C, add 50% sulfuric acid to acidify the solution to pH=3-4;
[0044] d. Add 300 ml of toluene and stir evenly, filter, and wash the solid with a small amount of toluene;
[0045] e. the filtrate is left to stand, after the organic phase is separated, the water phase is extracted with toluene, and the organic phase is combined;
[0046] f. Wash the organic phase with water to neutrality, remove toluene by rotary evaporator to obtain the crude product, and distill the cr...
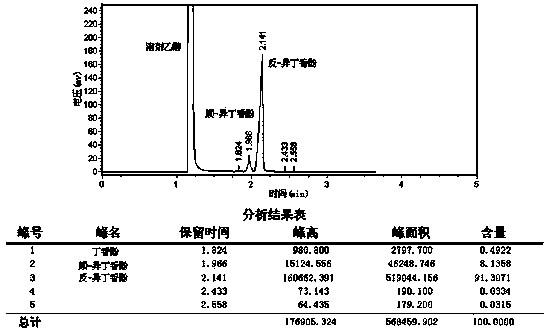
Embodiment 2
[0050] A synthetic method for isoeugenol, comprising the following steps:
[0051] a. Add 70.0 grams of 1,2-propanediol and 26.0 grams of potassium hydroxide in sequence in the three-necked bottle, stir and mix thoroughly, add 30.0 grams of eugenol under nitrogen protection, and continue to stir and mix;
[0052] b. Rapidly raise the temperature of the reactant to 165°C within 20 minutes under the protection of nitrogen, and reflux for 8 hours. This reaction is a closed reaction;
[0053] c. Cool down to 50-60°C, add 50% sulfuric acid to acidify the solution to pH=3-4;
[0054] d. Add 200 ml of toluene and stir evenly, filter, and wash the solid with a small amount of toluene;
[0055] e. the filtrate is left to stand, after the organic phase is separated, the water phase is extracted with toluene, and the organic phase is combined;
[0056] f. Wash the organic phase with water to neutrality, and remove the toluene by a rotary evaporator to obtain a crude product, which is t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com