Preparation method of 1,2,3-trimethoxy-5-allylbenzene
A technology of trimethoxybenzene and allylbenzene, which is applied in the direction of ether preparation, ester reaction to prepare ether, isomerization to prepare ether, etc., can solve the problems of difficult industrial mass production, high cost, and few reaction steps, and achieve The effect of low price, low cost and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Step a: Add 126 g of pyrogallic acid, 300 g of water and stir in the reaction bottle, 328 ml of 30% liquid caustic soda, add 310 ml of dimethyl sulfate dropwise at about 40°C, and the internal temperature of the reaction should not exceed 60°C, pH≥9. After the dropwise addition, heat up to 90°C and keep it warm for 1 hour, cool, separate the liquid, extract the precipitated solid with toluene in the water layer, filter and separate it, then distill it under reduced pressure, collect the fraction at 130-132°C (20mmHg), and obtain a white crystalline solid with a yield of 90%. .
[0038] Step b: Add 800ml of dichloromethane and 400g of aluminum trichloride to the reaction bottle, and dropwise add a dichloromethane solution of 1,2,3-trimethoxybenzene under stirring (168g of 1,2,3-trimethoxybenzene dissolves In 200ml of dichloromethane), the temperature is controlled at 20-40°C during the dropwise addition, and the stirring is continued for 2-3 hours after the dropwise add...
Embodiment 2
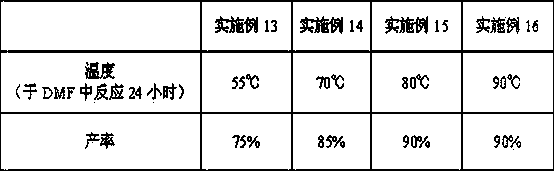
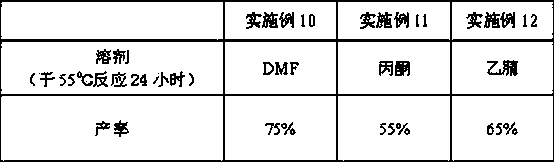
[0043] The selective demethylation reagent of step b is changed to AlCl 3 , other conditions are reacted under the same conditions as in Example 1, and the results are shown in Table 1.
Embodiment 3
[0045] The selective demethylation reagent in step b was changed to methanesulfonic acid, and the reaction was carried out under the same conditions as in Example 1, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com