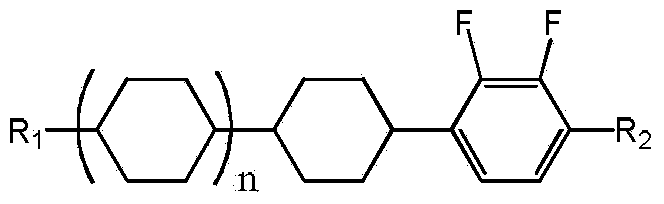
Conversion method of 1-cyclohexyl-2,3-difluorobenzene derivative cis-trans-isomers
A technology of difluorobenzene derivatives and cis-isomers, applied in the field of 1-cyclohexyl-2, can solve the problems of increasing product cost, increasing production cost, and difficult disposal of waste liquid, so as to avoid direct discharge and reduce Pollution, the effect of increasing production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
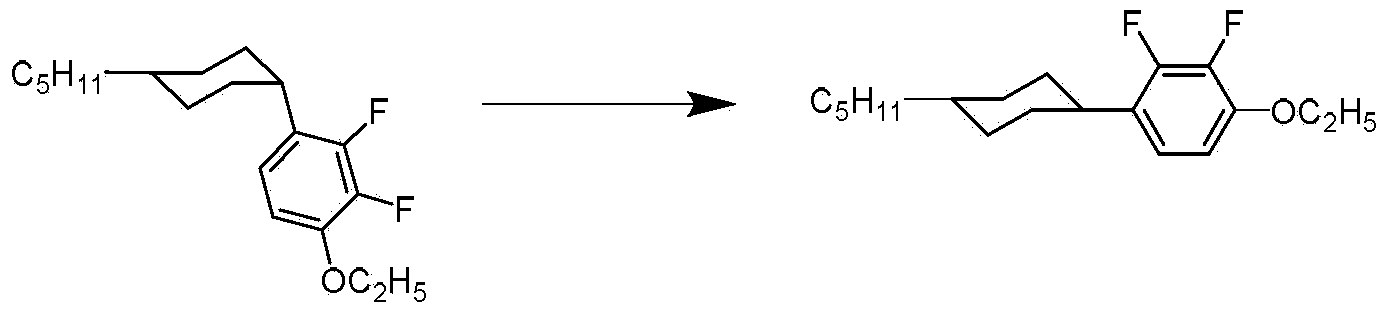
[0038] Example 1 trans 1-ethoxy-2,3-difluoro-4-(4-pentylcyclohexyl)benzene (R 1 is pentyl, R 2 is ethoxy, n is 0)
[0039]
[0040] In the synthesized 1-ethoxy-2,3-difluoro-4-(4-pentylcyclohexyl)benzene, the molar percentage of the cis isomer is 82%, and that of the trans isomer The mole percentage is 17%.
[0041] The method for converting the cis isomer to the trans isomer is as follows.
[0042] In a 250ml there-necked flask, add 25g1-ethoxy-2,3-difluoro-4-(4-pentylcyclohexyl)benzene, catalyst palladium carbon Pd / C (mass percentage of Pd) of 1.5g in dry weight The content is 5%), install the thermometer, stir, install a Y-shaped tube on one of the three-necked bottles, one port of the Y-shaped tube is connected to the trachea and the other is filled with a balloon. Fill the three-necked flask with nitrogen, vent the air with nitrogen for 5 times, and then vent the nitrogen with hydrogen for 5 times, and keep the balloon in an inflated state. At this time, the pressur...
Embodiment 2
[0051] Example 2 trans 1-ethoxy-2,3-difluoro-4-(4-propylcyclohexyl)benzene (R 1 is propyl, R2 is ethoxy, n is 0)
[0052]
[0053] In the synthesized 1-ethoxy-2,3-difluoro-4-(4-propylcyclohexyl)benzene, the molar percentage of the cis isomer is 85%, and that of the trans isomer The mole percentage is 14%.
[0054] The method for converting the cis isomer to the trans isomer is as follows.
[0055] Add 25g of 1-ethoxy-2,3-difluoro-4-(4-propylcyclohexyl)benzene into a 250ml three-necked flask, the dry weight is 5gPt / C (the mass percentage of Pt is 5% ), and ethanol 10g, water 10g, dioxane 5g. Install the thermometer, stir, install a Y-shaped tube on one mouth of the three-necked bottle, and connect one mouth of the Y-shaped tube to the trachea and install a balloon on the other mouth. Fill the three-necked flask with nitrogen, vent the air with nitrogen for 5 times, and then vent the nitrogen with hydrogen for 5 times, and keep the balloon in an inflated state. At this ti...
Embodiment 3
[0064] Example 3 trans 4'-(4-methyl-2,3-difluorobenzene)-4-propyl bicyclohexane (R 1 is propyl, R 2 is methyl, n is 1)
[0065] In the synthesized 4′-(4-methyl-2,3-difluorobenzene)-4-propylbicyclohexane, the molar percentage of the cis-isomer is 90%, and the trans-isomer The mole percent content is 9%.
[0066] The method for converting the cis isomer to the trans isomer is as follows.
[0067] In the 500ml autoclave, add the 4 '-(4-methyl-2,3-difluorobenzene)-4-propyl bicyclohexane of 50g, the palladium charcoal that dry weight is 0.125g (the mass percent composition of Pd is 5%), install the lid of the kettle, keep the airtightness good, and install the thermometer. Fill the autoclave with nitrogen to exhaust the air 5 times, then use hydrogen to exhaust the nitrogen 5 times, then fill the autoclave with hydrogen to a pressure of 5.0 atm, start stirring, heat to 240 ° C, and carry out the catalytic reaction for 1 hour.
[0068] The reaction liquid was sampled and analyz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com