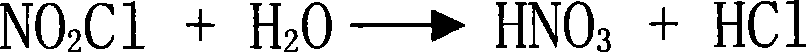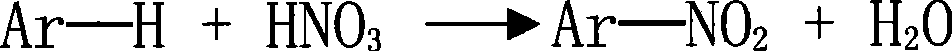Method for preparing aromatic nitro compound using nitryl chloride as nitrating agent
A technology for aromatic nitro group and nitroxyl chloride is applied in the field of nitration of aromatic hydrocarbons and their derivatives, which can solve the problems of difficult treatment of nitroxyl chloride, and achieve the effects of saving funds, saving investment and reducing energy consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] Example 1: Preparation of nitrotoluene by nitration of toluene
[0021] Production according to the following specific steps: 1) Toluene, 69% sulfuric acid and nitroxyl chloride are respectively sent to the nitration reactor through a rotameter for nitration reaction, the mass ratio of toluene and 69% sulfuric acid is 1:2.9, and the reaction temperature is 20°C. Normal pressure, when the reaction finishes, the content of toluene in the feed liquid is less than 4%, and the content of dinitrotoluene is less than 0.2%;
[0022] 2) After the nitration reaction is finished, the feed liquid enters the separator, and the separated mononitrotoluene is washed with water, alkali washed, washed with water and distilled to obtain the industrial product mononitrotoluene; the separated concentration is 69% and waste acid is returned to the nitration The reactor is recycled; the molar ratio of the obtained mononitrotoluene mixture is: o-nitrotoluene: p-nitrotoluene: m-nitrotoluene=60:...
example 2
[0023] Example 2: Preparation of nitrochlorobenzene by nitration of chlorobenzene
[0024] Production according to the following specific steps: 1) Chlorobenzene, 70% sulfuric acid and nitroxyl chloride are respectively sent to the nitration reactor through the flow rate for nitration reaction, the mass ratio of chlorobenzene and 70% sulfuric acid is 1:2.3, and the reaction temperature is 55°C , normal pressure, the content of chlorobenzene in the feed liquid is not more than 3%, and the content of polynitrochlorides is not more than 0.2% when the reaction ends;
[0025] 2) After the nitration reaction is finished, the feed liquid enters the separator, and the organic matter in the upper layer enters the neutralization pot to add alkali for neutralization, water washing, and drying tower treatment; 70% of the waste acid in the lower layer of the separator is returned to the nitration reactor for recycling;
[0026] 3) The organic matter obtained by the reaction is mainly nitro...
example 3
[0027] Example 3: Preparation of nitrobenzene by nitration of benzene
[0028] Production according to the following specific steps: 1) Send benzene, 71% sulfuric acid and nitroxyl chloride into the nitration reactor through a flow meter for nitration reaction, the mass ratio of benzene and 71% sulfuric acid is 1:3.3, and the reaction temperature is 60 ° C. When the reaction finishes, the content of benzene in the feed liquid is not more than 3%, and the content of dinitrobenzene is not more than 0.2%;
[0029] 2) After the nitration reaction is finished, the feed liquid enters the separator, and the separated nitrobenzene is washed with water, alkali washed, washed with water and distilled to obtain the industrial product nitrobenzene; the separated concentration is 71% and waste acid is returned to the nitration reactor recycle. The reaction yield can reach 99% in terms of benzene, and the product content is not less than 99.5%
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com