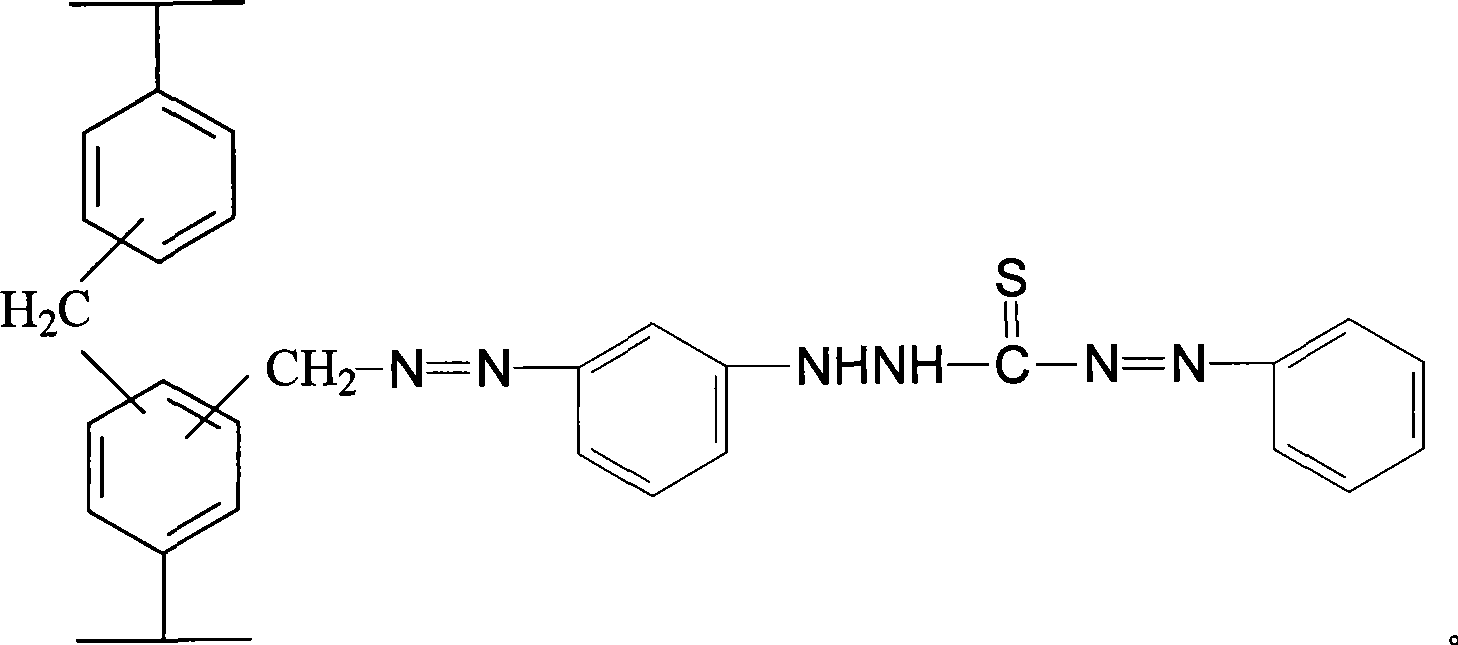
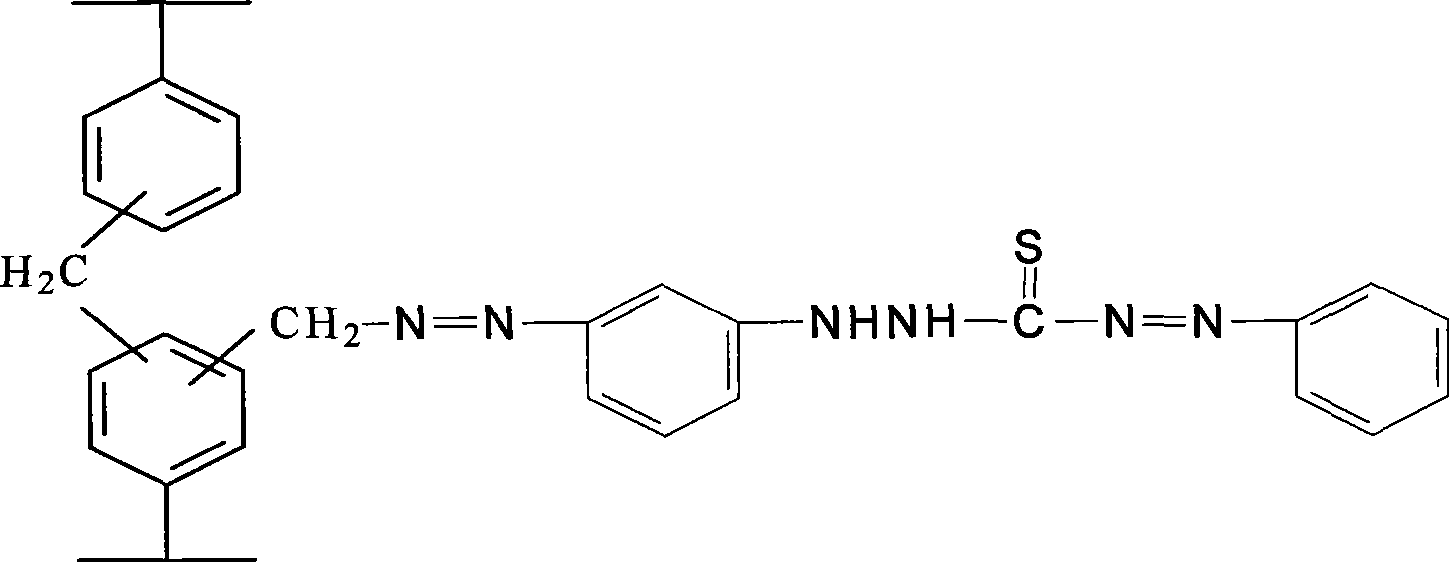
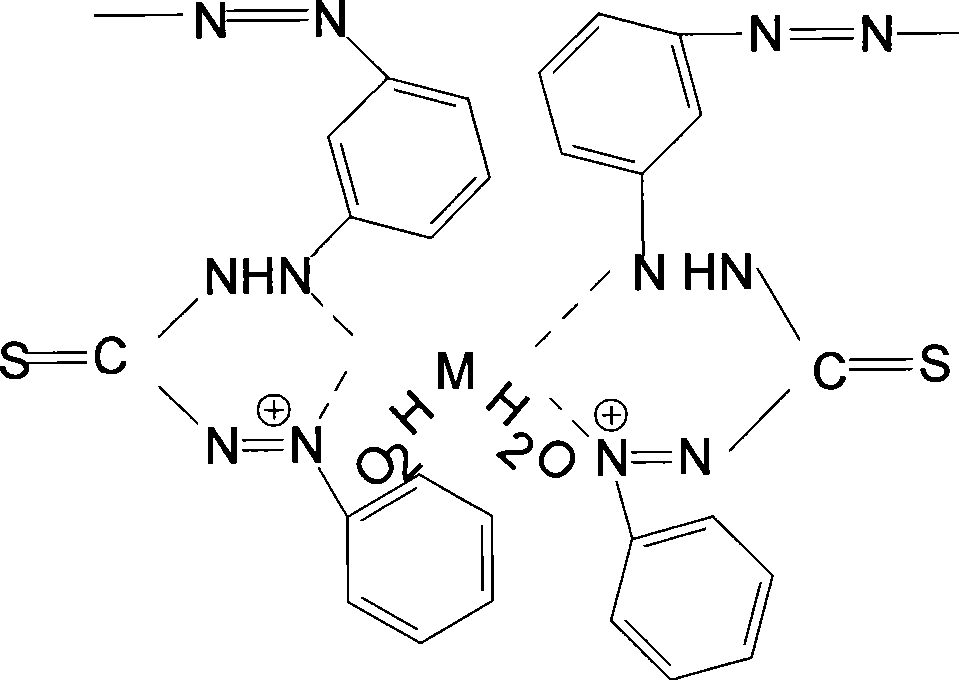
Linking dithizone resin and preparation method thereof
A technology of bonding dithizone and dithizone, which is applied in chemical instruments and methods, and other chemical processes, can solve the problems of unreported synthesis, achieve wide application prospects and improve stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (a) Suspension polymerization.
[0026] Add a certain amount of dilute sodium hydroxide solution to the styrene monomer or directly filter the monomer through a resin column equipped with a strong base anion exchange resin to remove the polymerization inhibitor.
[0027] In a 1000mL beaker, add 210g styrene, 26g divinylbenzene, and 140g liquid paraffin, stir well and set aside.
[0028] In a 1000mL three-neck flask, add 600g of distilled water and raise the temperature to 40°C, then add 6g of gelatin, stir until completely dissolved, and at the same time raise the temperature to 50°C, add 2mL of methylene blue, stir well, add the above-mentioned styrene, divinylbenzene and liquid Add the initiator benzoyl peroxide 4.0g to the wax mixture; adjust the stirring speed to make the oil droplet size appropriate, gradually raise the temperature to 80°C at a speed of 1°C / 6min, keep it warm for 4 hours, and then gradually raise the temperature to 85°C Keep warm for 3 hours, keep...
Embodiment 2
[0036] The synthetic steps of another embodiment of the present invention are as follows:
[0037] (a) Utilize styrene as a monomer, divinylbenzene as a crosslinking agent, utilize No. 200 solvent oil as a porogen, use gelatin (or polyvinyl alcohol, or several mixtures of magnesium carbonate, gelatin, and polyvinyl alcohol) ) as dispersant, using benzoyl peroxide as initiator, adopting suspension polymerization method to synthesize macroporous polystyrene-divinylbenzene copolymer with low crosslinking degree, select ethanol (or acetone, industrial alcohol, low boiling point solvent gasoline) ) for solvent extraction to remove the porogen remaining in the resin channels, and dry to obtain low-crosslinked macroporous polystyrene-divinylbenzene resin, that is, white balls.
[0038] Wherein the dosage of the crosslinking agent is 7% of the total amount of the monomer and the crosslinking agent, the dosage of the porogen is 100% of the weight of the monomer, and the crosslinking de...
Embodiment 3
[0046] The synthetic steps of another embodiment of the present invention are as follows:
[0047] (a) Using styrene as a monomer, divinylbenzene as a crosslinking agent, using liquid wax as a porogen, using magnesium carbonate and polyvinyl alcohol as a dispersant, using benzoyl peroxide as an initiator, and adopting suspension polymerization Synthesize macroporous polystyrene-divinylbenzene copolymer with low cross-linking degree, use steam distillation or ethanol as solvent extraction to remove the porogen remaining in the resin channels, and dry to obtain macroporous polystyrene with low cross-linking degree. Ethylene-divinylbenzene resin, that is, white balls.
[0048] Wherein the dosage of the crosslinking agent is 4% of the total amount of the monomer and the crosslinking agent, the dosage of the porogen is 80% of the weight of the monomer, and the crosslinking degree of the low crosslinking macroporous polystyrene is 8%.
[0049] (b) In a 1000ml three-neck flask, add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com