Synthesis method of alpha-nitrocycloalkanone
The technology of a nitrocycloalkanone and a synthesis method, which is applied in the field of organic intermediate synthesis, can solve the problems of no compound, expensive organotin compound and the like, and achieve the effect of simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
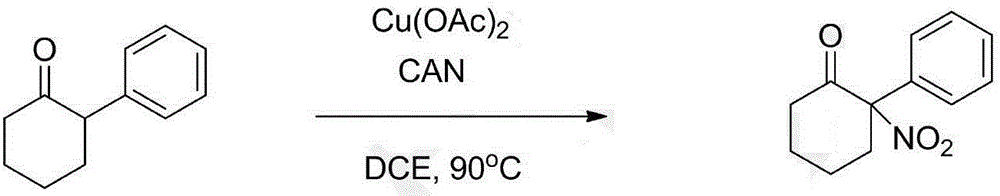
[0019] Using 2-phenylcyclohexanone as raw material
[0020]
[0021] 2-Phenylcyclohexanone (34.8mg, 0.2mmol), cerium ammonium nitrate (330.0mg, 0.6mmol) and Cu(OAc) were sequentially added into a dry sealed 15mL tube 2 (16mg, 0.10mmol), then add 2.0mL DCE under argon atmosphere, seal with a lid, and react in an oil bath at 120°C for 12 hours. When the raw materials were consumed, the system was cooled to room temperature, then diluted with 5.0 mL of petroleum ether, and directly subjected to column chromatography (petroleum ether / ethyl acetate=40:1). A pale yellow solid (25.8 mg, yield 51.0%) was finally obtained.
[0022] The product detection data are as follows:
[0023] 1 H NMR (400MHz, CDCl 3 ):δ7.48-7.44(m,3H),7.36-7.33(m,2H),3.10-3.03(m,1H),2.92-2.86(m,1H),2.70-2.63(m,1H),2.59 -2.53(m,1H),1.97-1.87(m,3H),1.82-1.73(m,1H); 13 C NMR (100MHz, CDCl 3 ): δ200.5, 132.1, 130.2, 129.2, 128.4, 101.0, 40.2, 35.3, 27.3, 22.1.
Embodiment 2
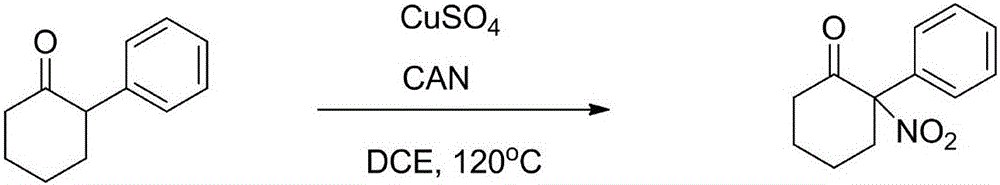
[0025] Using 2-phenylcyclohexanone as raw material
[0026]
[0027] Add 2-phenylcyclohexanone (34.8mg, 0.2mmol), ammonium cerium nitrate (495.0mg, 0.9mmol) and CuSO 4 (6.4mg, 0.04mmol), then added 4.0mL DCE under argon atmosphere, sealed with a lid, and reacted in an oil bath at 120°C for 16 hours. When the raw materials were consumed, the system was cooled to room temperature, then diluted with 6.0 mL of petroleum ether, and directly subjected to column chromatography (petroleum ether / ethyl acetate=40:1). A pale yellow solid (20.1 mg, yield 46.0%) was finally obtained.
[0028] The spectral data of the product are the same as above.
Embodiment 3
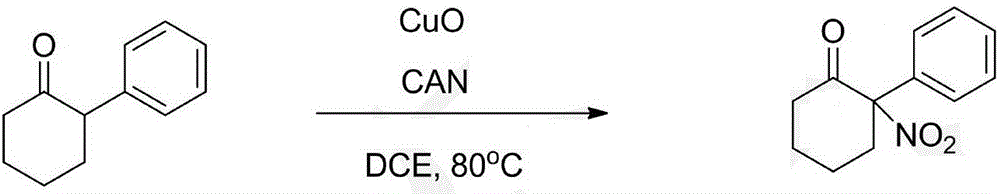
[0030] Using 2-phenylcyclohexanone as raw material
[0031]
[0032] 2-Phenylcyclohexanone (34.8mg, 0.2mmol), cerium ammonium nitrate (495.0mg, 0.9mmol) and CuO (9.54mg, 0.06mmol) were successively added to a dry 15mL sealed tube, and then the Add 6.0mL DCE, seal with a lid, and react in an oil bath at 120°C for 10 hours. When the raw materials were consumed, the system was cooled to room temperature, then diluted with 6.0 mL of petroleum ether, and directly subjected to column chromatography (petroleum ether / ethyl acetate=40:1). A pale yellow solid (23.2 mg, yield 52.9%) was finally obtained.
[0033] The spectral data of the product are the same as above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com