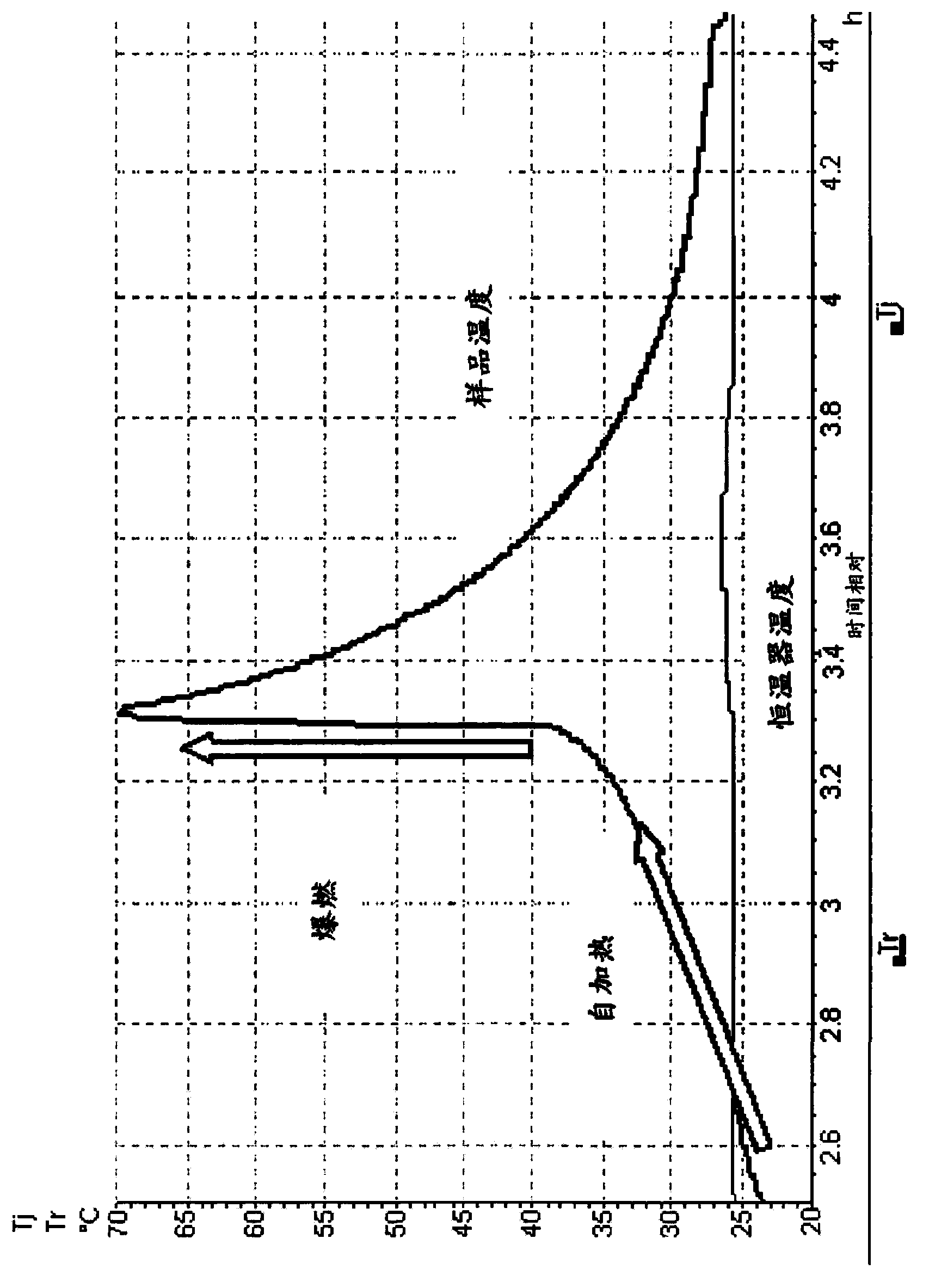
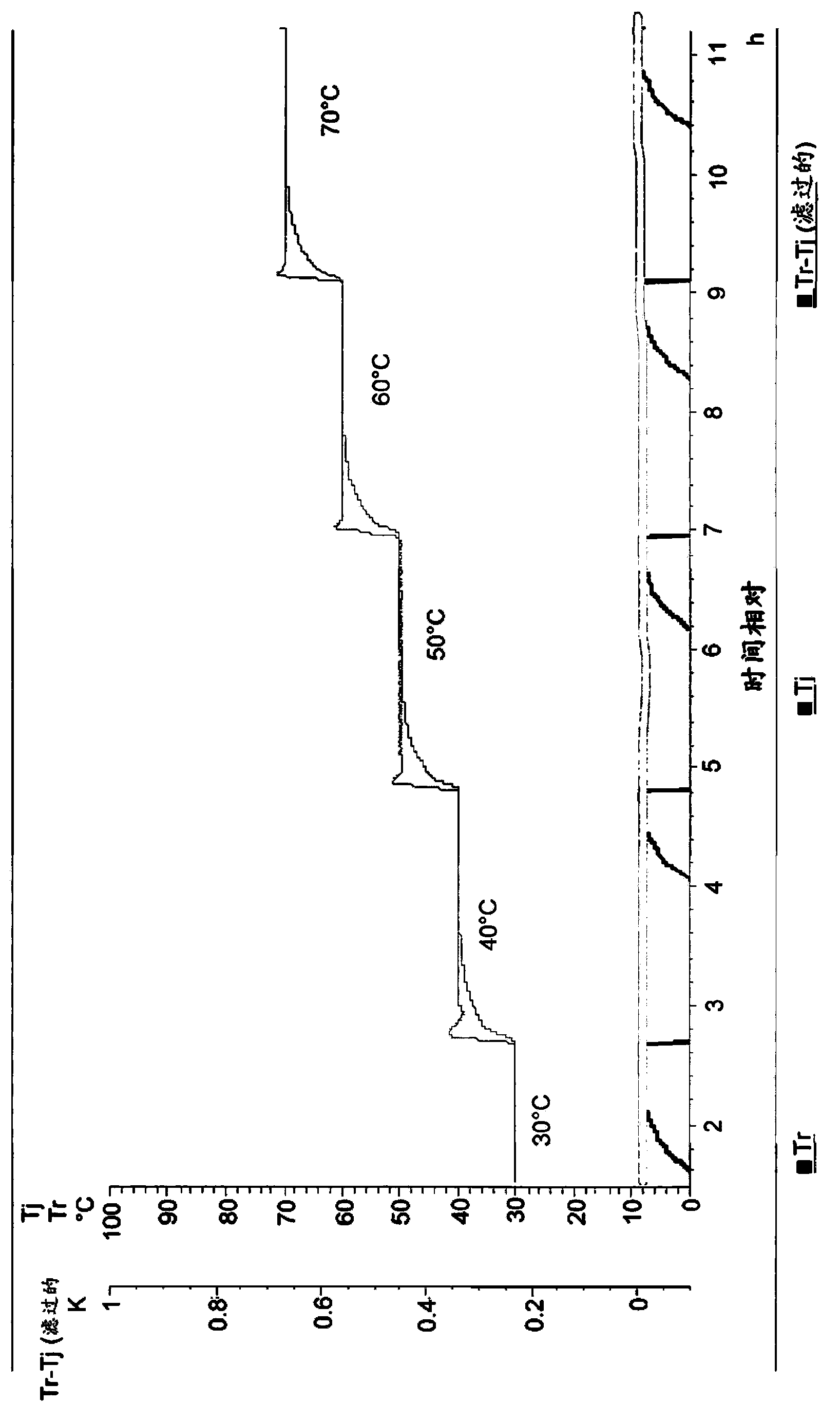
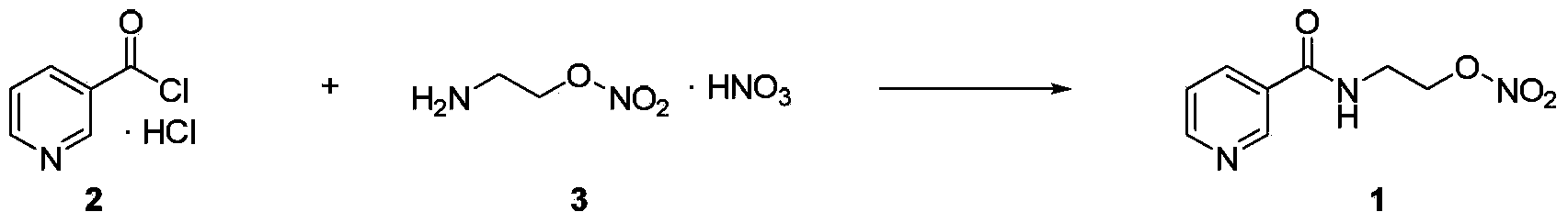Process for the manufacture of nicorandil
A technology of nicorandil and nitric acid, applied in the field of preparing nicorandil, can solve problems such as safety hazards, unfavorable economic and industrial safety, large processing volume and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Synthesis of Nicorandil
[0058] A 100 mL three-neck reaction flask purged with nitrogen and equipped with an anchor magnetic stirrer, temperature probe and dropping funnel was charged with 6.00 g of glacial acetic acid, followed by the addition of 1.35 g of 68% nitric acid. The system was thermostated at a temperature of 22°C, and 4.00 g of acetic anhydride was added within about 15 minutes. Stir the system for about 15 minutes; then add 1.00 g of N-(2-hydroxyethyl)nicotinamide in batches within ≈15 minutes, and the temperature does not exceed 22°C. The system was stirred at 22°C for 30 minutes, then the progress of the reaction was checked by HPLC. N-(2-hydroxyethyl)nicotinamide content <1%. The contents of the flask were slowly poured into 24% aqueous ammonia (14.0 g), taking care that the temperature did not exceed 35 °C. The temperature was lowered to 5°C and held for about 30 minutes. The solid was isolated by filtration and washed with 1 mL of water.
[0059...
Embodiment 2
[0061] initial magnification
[0062] 300 g of glacial acetic acid was charged to a 1000 mL three-neck reaction flask purged with nitrogen and equipped with an anchor magnetic stirrer, temperature probe and dropping funnel, followed by the addition of 67.5 g of 68% nitric acid. The system was kept at a temperature of 18°C, and 200 g of acetic anhydride was added within about 45 minutes, being careful not to exceed 25°C and stopping the addition if it exceeded this threshold. The reaction is very rapid, so the reaction can be controlled by reagent addition. The system was stirred for about 30 minutes; then cooled to 15°C, and 50.0 g of N-(2-hydroxyethyl)nicotinamide was added in five identical 10.0 g portions at 15 minute intervals, after each addition Take care to return the temperature to 15°C before. The system was stirred at 22°C for 90 minutes, then the progress of the reaction was checked by HPLC. N-(2-hydroxyethyl)nicotinamide content <1%. The contents of the flask w...
Embodiment 3
[0065] Synthesized by sulfuric acid / nitric acid mixture (reaction time 90 minutes; temperature 10°C)
[0066] 18.4 g of concentrated sulfuric acid was charged into a 100 mL three-neck reaction flask purged with nitrogen and equipped with an anchor magnetic stirrer, temperature probe and dropping funnel. The mixture was cooled to 0°C, and then 16.9 g of 68% nitric acid was added dropwise, taking care in view of the strongly exothermic reaction. The system was kept at a constant temperature of 10° C., and 10.0 g of N-(2-hydroxyethyl)nicotinamide was added within about 5 minutes to maintain the temperature. The system was stirred for 30 minutes, then the conversion was checked by HPLC. The residual N-(2-hydroxyethyl)nicotinamide content was 33.5%. The reaction mixture was colorless. The mixture was left under stirring at 10°C for a further 1 hour before checking for conversion again. The residual N-(2-hydroxyethyl)nicotinamide content was 25.3%. The reaction mixture turned t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com