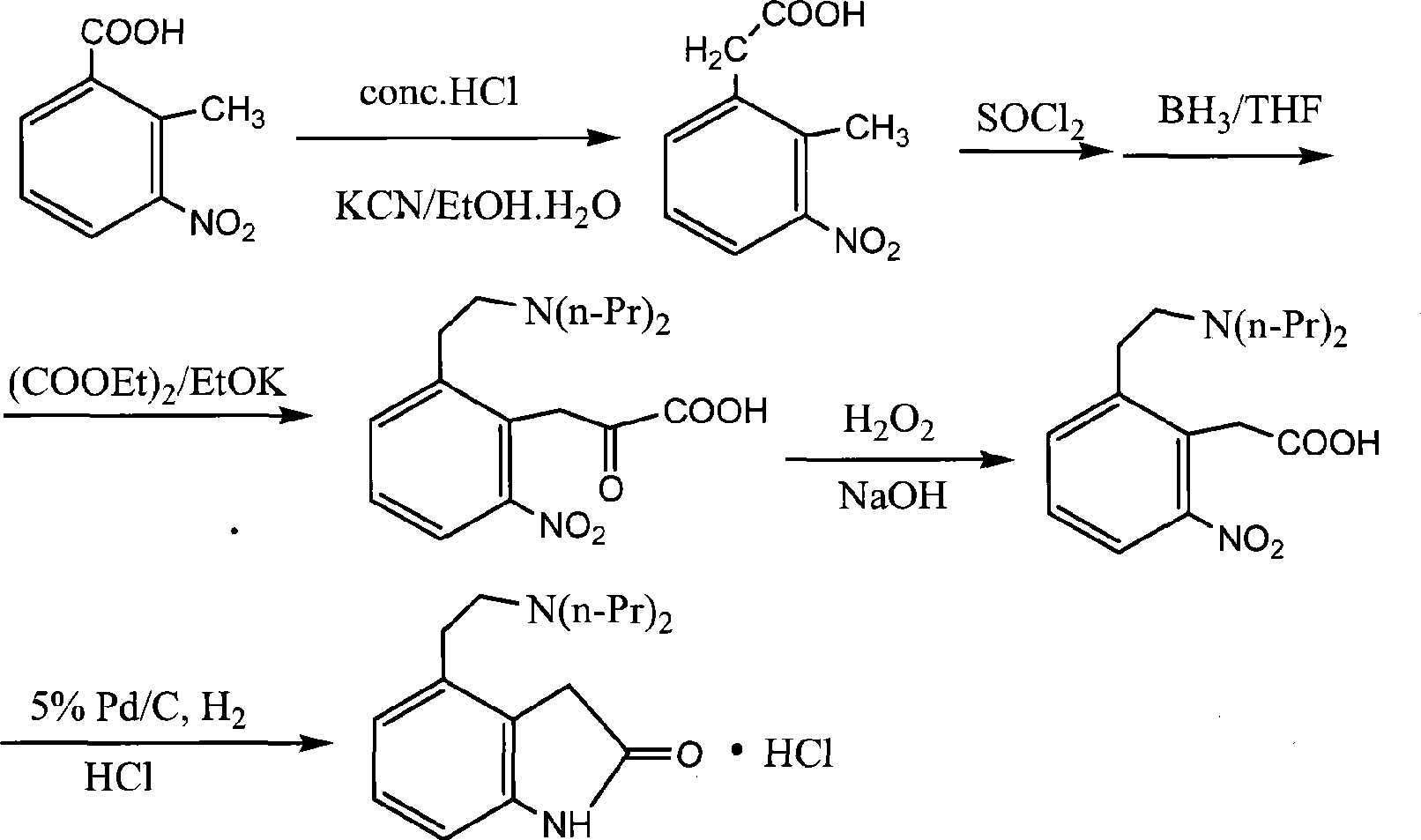
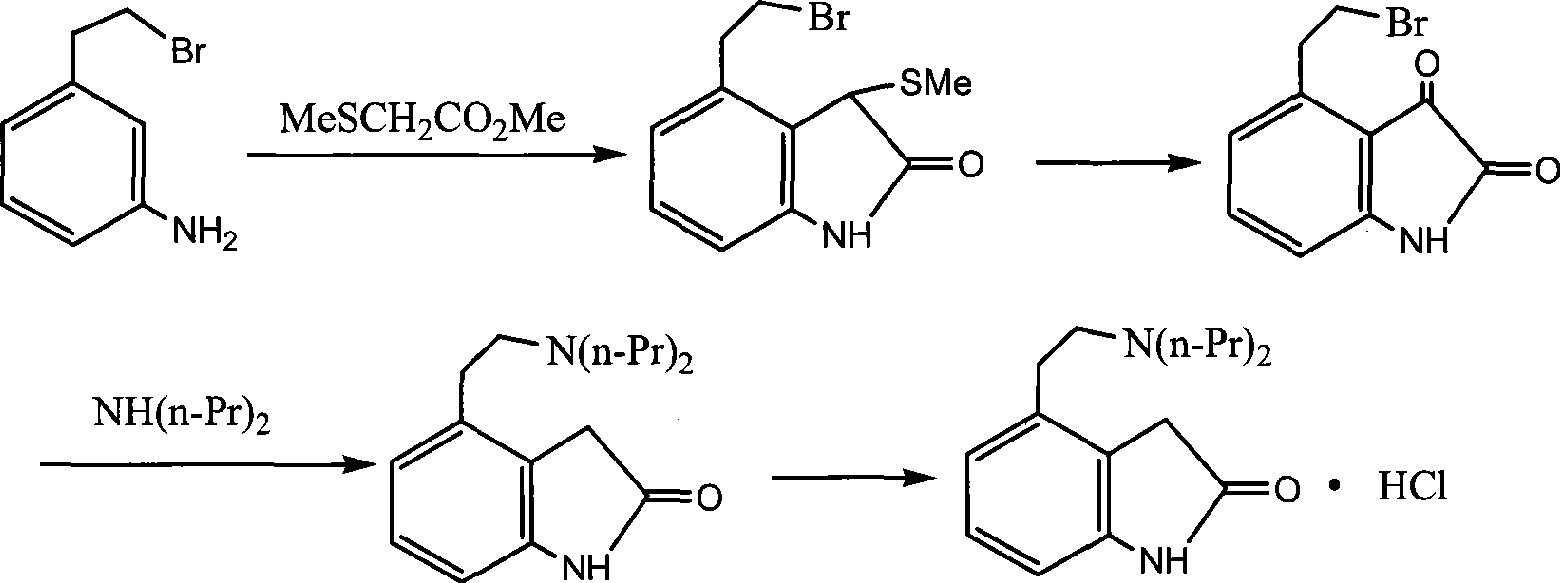
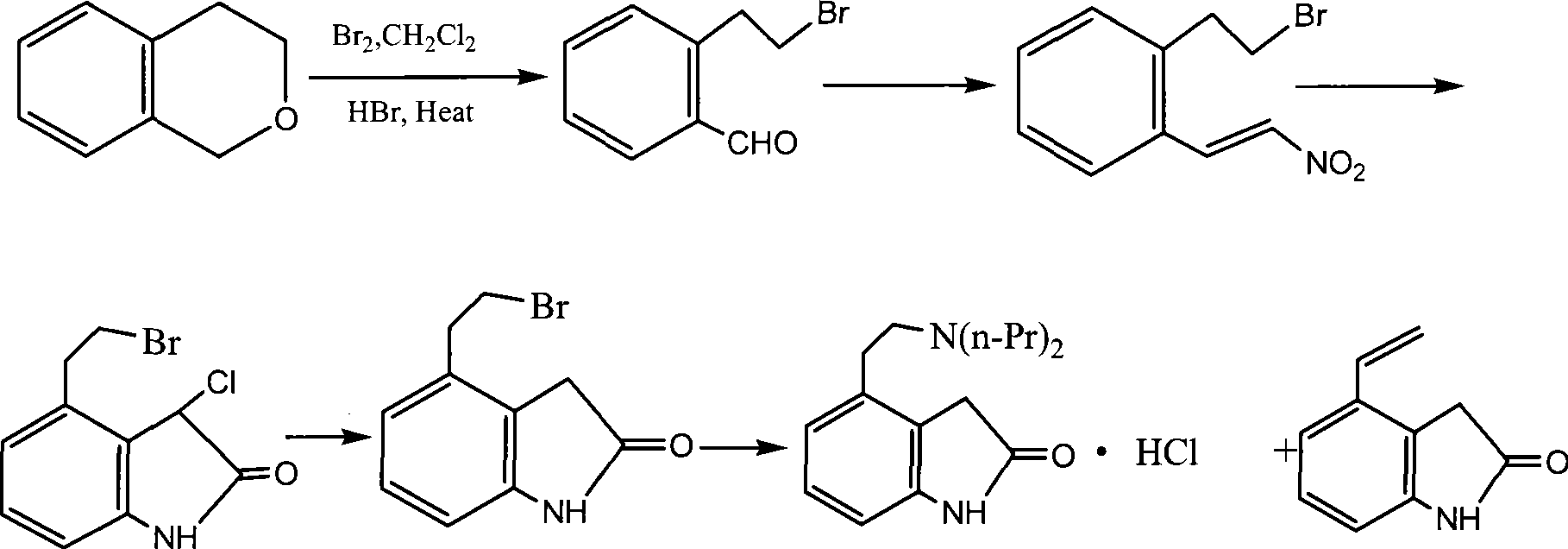
Preparation of ropinirole hydrochloride
A technology of ropinirole hydrochloride and acidic conditions, which is applied in the field of preparation of ropinirole hydrochloride, can solve the problems of harsh reaction conditions, low reaction yield, and difficulty in purchasing, and achieves mild production conditions, easy availability of raw materials, Easy to use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0038] Specific embodiments of the present invention will be described below, but not limited to this embodiment.
[0039] A preparation method of ropinirole hydrochloride, comprising the following steps in turn:
[0040] (1), synthetic intermediate (I) isochroman: in reaction flask, add β-phenethyl alcohol 35ml, paraformaldehyde 9.5g, vitriol oil 26ml, stir, be heated to 40~45 ℃, insulation reaction After 3 hours, cooling, standing to separate layers, the aqueous layer was extracted with dichloromethane, the organic layers were combined, washed, and the solvent was distilled off under reduced pressure to obtain intermediate (I).
[0041] (2), synthetic intermediate (II) 2-bromoethylbenzaldehyde: add intermediate (I) 67.5g and methylene dichloride 150ml in reaction flask, drip bromine 80g, temperature of reaction is controlled at 40~45 ℃ , after the dropwise addition was completed, the temperature was raised to 80° C., and after 1 hour of heat preservation, the mixture was co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com