Synthesis method of (E)-beta-nitrostyrene
A technology of nitrostyrene and a synthesis method, which is applied in the field of synthesis of -β-nitrostyrene, can solve the problems of difficult industrial application, low reaction efficiency, low yield and the like, and achieves the advantages of being beneficial to industrial production, simplifying Process steps, effect of high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
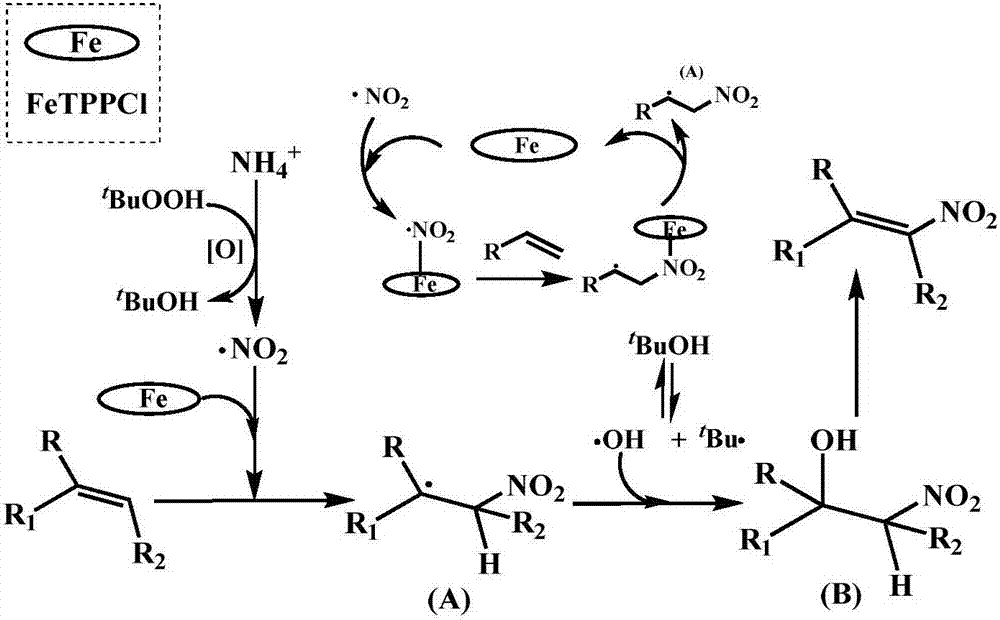
[0038] Styrene (0.5mmol), NH 4 I (1.5 equivalents, 0.75mmol, 108mg), TBHP (70% H 2 O, 6.0 equiv, 3.0 mmol, 384 mg), TPPFeCl (3-5% on molar styrene) and acetonitrile (2 mL) were added to a sealed tube. Acetonitrile was added first, followed by styrene, NH 4 I and TPPFeCl, and finally TBHP. The reaction was stirred vigorously at 120 °C for 6 hours and monitored by TLC. After the reaction was complete, the mixture was cooled to room temperature, then filtered and washed with ethyl acetate (EA). Finally, the filtrate was concentrated with a rotary evaporator, and purified by column chromatography with silica gel (200-300 mesh) using petroleum ether (PE) / ethyl acetate (EA) as eluent.
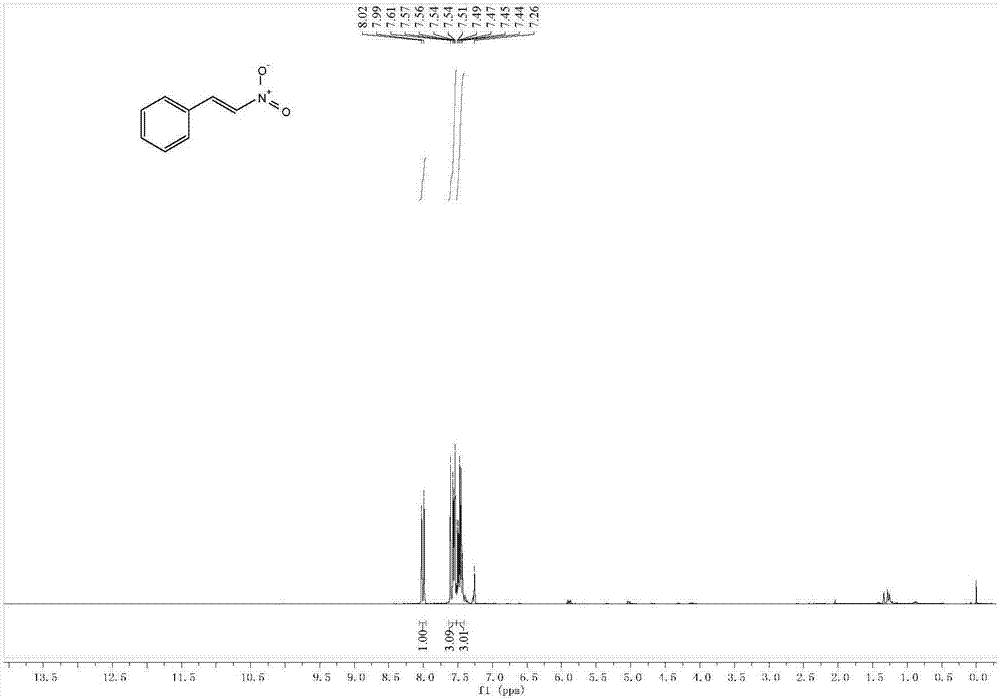
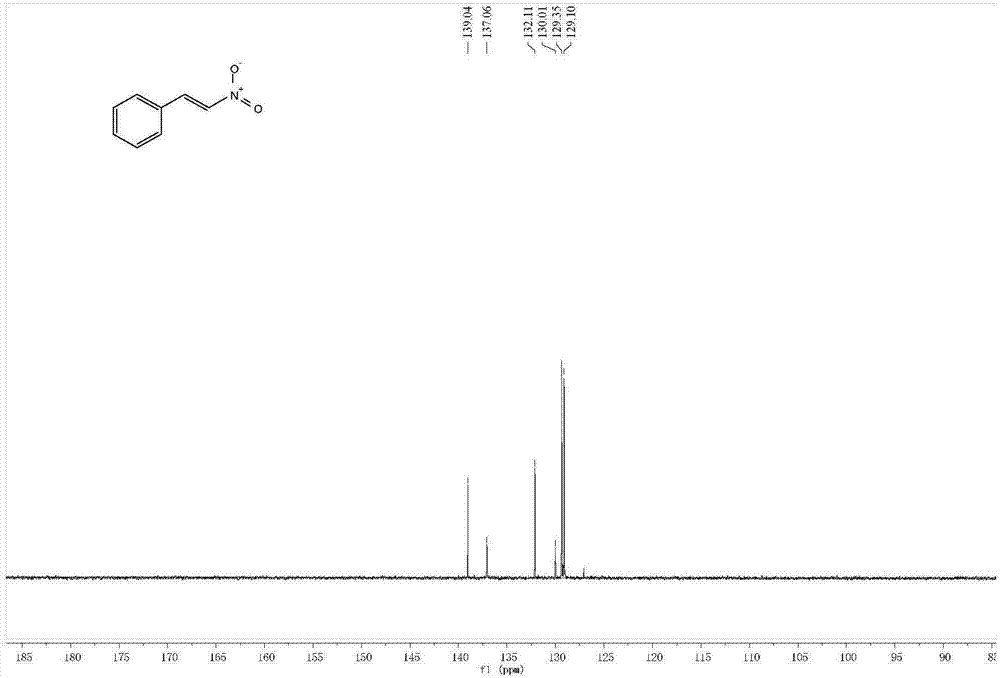
[0039] Yellow solid: 82% (61mg). 1 H NMR (400MHz, CDCl 3 )δ8.01(d, J=13.7Hz, 1H), 7.63–7.53(m, 3H), 7.47(tdd, J=8.5, 5.2, 3.6Hz, 3H). 13 C NMR (101MHz, CDCl 3 )δ139.04,137.06,132.11,130.01,129.35,129.10.GC-MS(m / z):149.+.
[0040] Compared with experimental group 1:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com