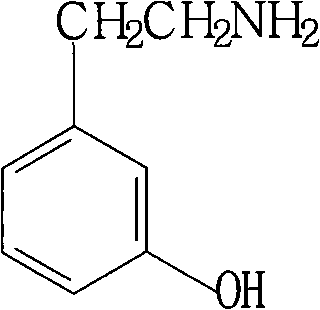
Method for synthesizing m-hydroxyphenylacetamide
A technology for m-hydroxyphenethylamine and hydroxyphenethylamine, which is applied in the field of synthesis of hydroxyarylethylamine, can solve problems such as numerous reaction steps and greater environmental harm, and achieves fewer experimental steps, higher yield and lower cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
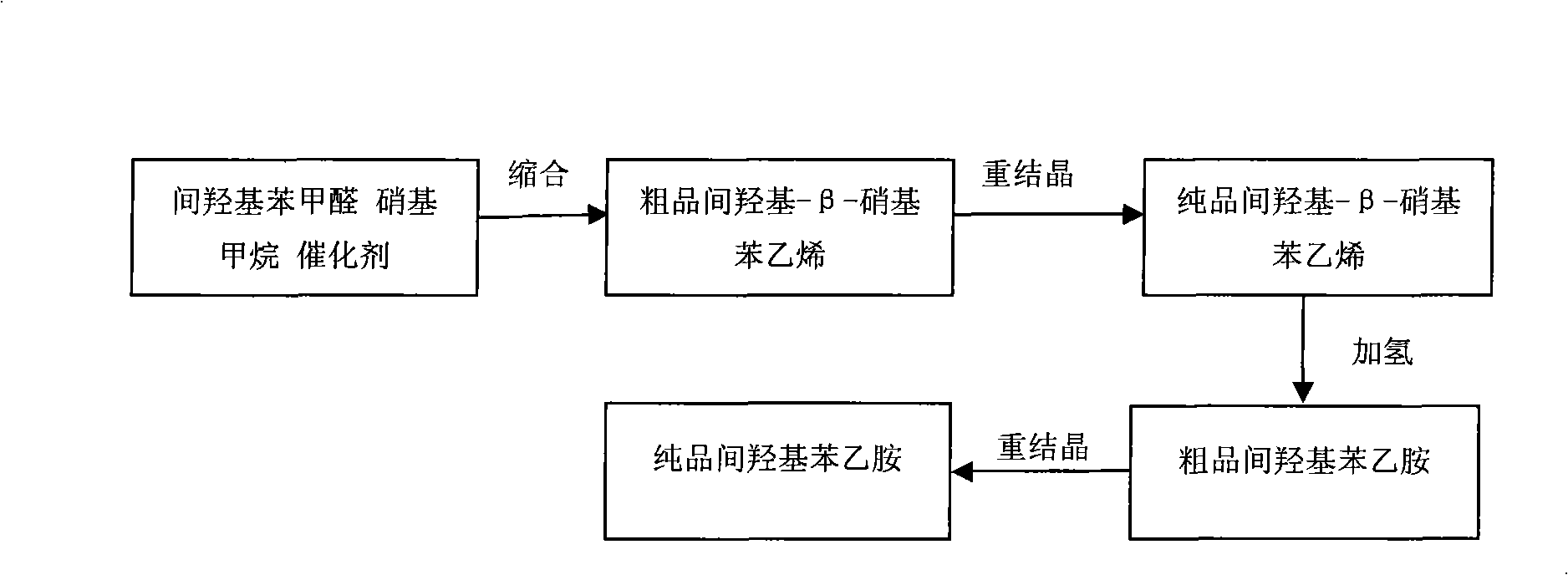
[0030] combine figure 1 , the synthetic method of m-hydroxyphenylethylamine of the present invention is to take m-hydroxybenzaldehyde as raw material to prepare m-hydroxyphenylethylamine through condensation, catalytic hydrogenation reduction, and its steps are as follows:
[0031] The first step, add solvent in m-hydroxybenzaldehyde and nitromethane, catalyst, carry out condensation reaction in reactor or reaction bottle; Wherein the solvent that adds is organic solvent, as ethyl acetate, dichloromethane, chloroform , tetrachloromethane, ether or petroleum ether. The mass ratio of m-hydroxybenzaldehyde to nitromethane is 1:1.5-1:1.7, and the amount of catalyst used is 20%-40% of the mass fraction of m-hydroxybenzaldehyde. The catalyst is a weak acid and weak base compound, such as NH 4 HCO 3 , NH 2 CH 2 CH 2 Oh. The condensation reaction temperature is 60°C-90°C, and the reaction time is 2h-6h.
[0032] In the second step, the reaction solution in the first step is po...
Embodiment 1
[0037] Embodiment 1: the synthesis of m-hydroxy-β-nitrostyrene
[0038] In a 250mL four-necked flask equipped with a magnetic stirrer, a thermometer and a reflux condenser, add m-hydroxybenzaldehyde (0.02mol), nitromethane (0.03mol), acetic acid (17mL) and ammonium acetate (0.007mol), Heat and stir, raise the temperature to 80°C, and keep warm for reaction. After the reaction was completed, the reaction system was cooled to room temperature, poured into an appropriate amount of ice water, and a yellow precipitate was precipitated. At this time, it is not necessary to filter, but directly heat the system to 60-70 ° C, and slowly add ethanol dropwise under stirring until the precipitate is completely dissolved, then pour the solution into an appropriate amount of ice water, and bright yellow needles are rapidly precipitated. crystals and sink to the bottom. Stand still for about 5 minutes. After the bright yellow crystals are precipitated, pour off the upper layer solution and...
Embodiment 2
[0039] Embodiment 2: the synthesis of m-hydroxyphenylethylamine
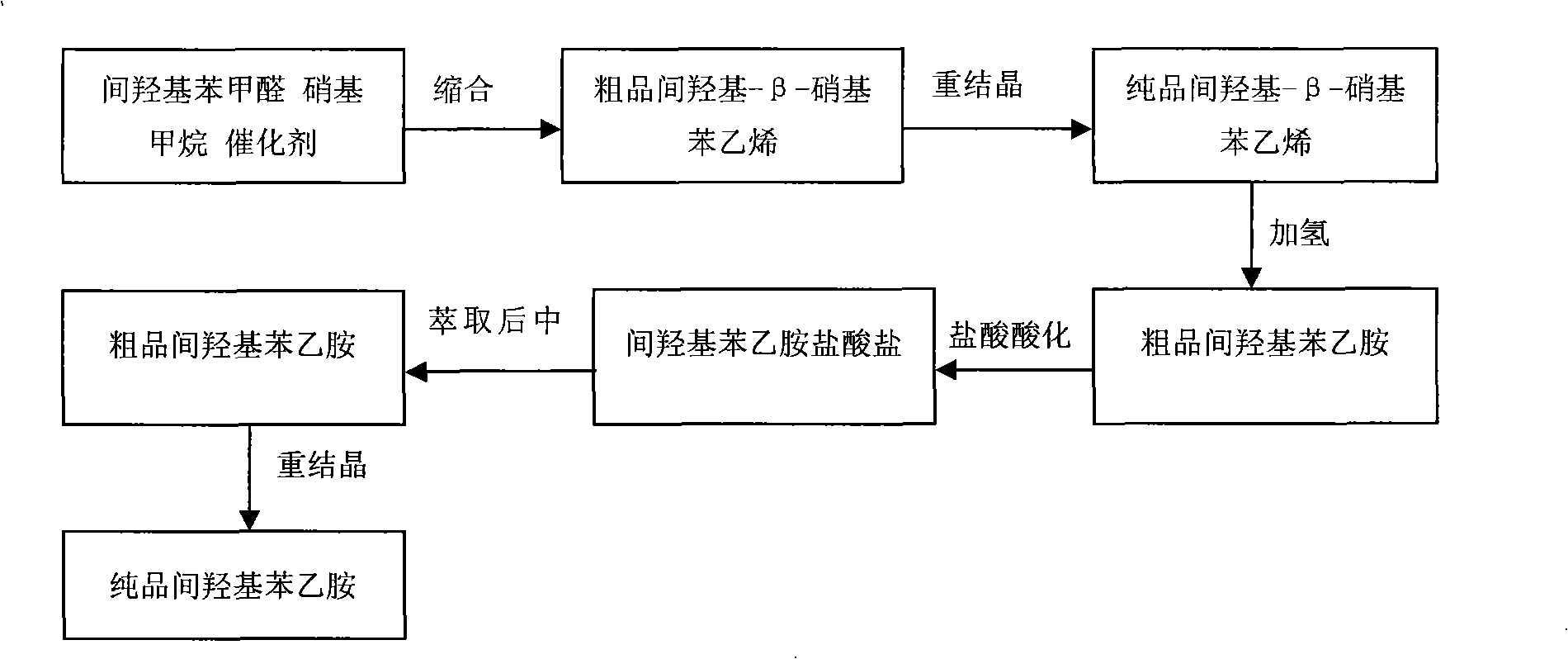
[0040] Dissolve an appropriate amount of m-hydroxy-β-nitrostyrene (50g) in a certain amount of methanol, put it into the hydrogenation reactor, add 0.5g5%Pd / C, feed nitrogen, replace 5 times, and then Hydrogen gas was introduced under stirring to make the pressure 2.5Mpa, then the temperature was raised to 100°C, the reaction was carried out for 2 hours, and the temperature was maintained for 1 hour. After the reaction is completed, ventilate, press filter, add an appropriate amount of hydrochloric acid solution to the reacted solution to make it acidic, and use CH 2 Cl 2 Extract, separate the water phase, and then add an appropriate amount of Na to the water phase 2 CO 3 The solution is made alkaline, distilled under reduced pressure, and then extracted with hot ethyl acetate, and the ethyl acetate solution is distilled under reduced pressure again to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com