Method for synthesizing multi-substituted pyrrole derivatives
A technology of pyrrole derivatives and synthetic methods, which is applied in the direction of organic chemistry, can solve problems such as increased synthesis costs and increased reaction safety risks, and achieve the effects of cost-saving synthesis, high yield, and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
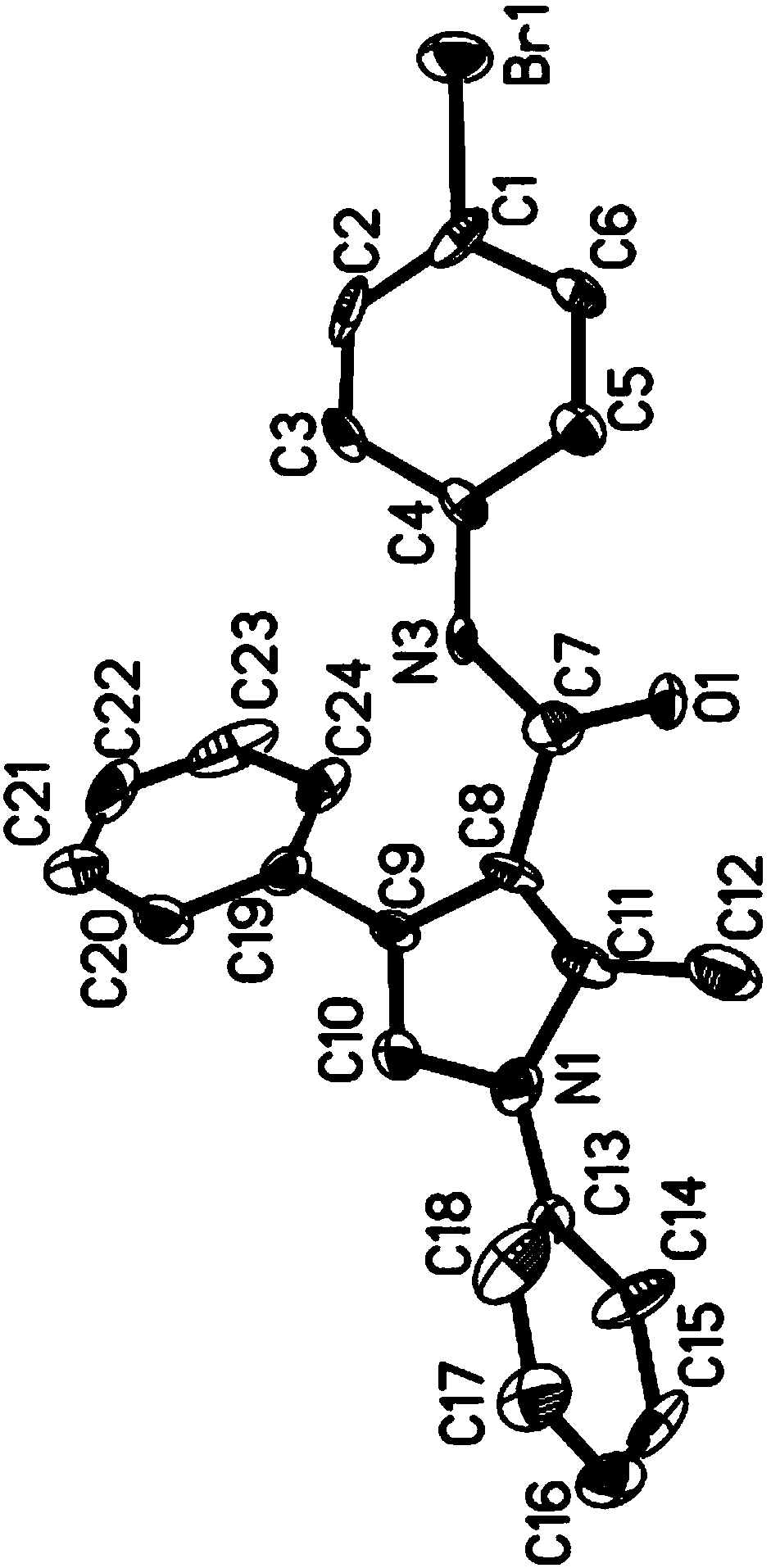
Image
Examples
Embodiment 1
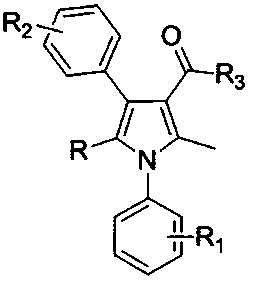
[0031] Example 1, a method for synthesizing polysubstituted pyrrole derivatives, the structure of the polysubstituted pyrrole derivatives is as described in formula I, in formula I:
[0032] R substituting group is selected from hydrogen, alkyl, aryl;
[0033] R 1 , R 2 The substituting group is selected from hydrogen, halogen, alkyl, alkoxy, aryl;
[0034] R 3 The substituting group is selected from alkyl, alkoxy, arylamino;
[0035] The synthesis method uses aromatic primary amine derivatives shown in formula II, β-nitrostyrene and its derivatives shown in formula III, and 1,3-dicarbonyl compounds shown in formula IV as raw materials, and uses LAuCl and AgOTf as catalysts. , react at 20°C to generate the polysubstituted pyrrole derivatives described in formula I; the specific steps are as follows:
[0036] (1) In the reaction vessel, add an appropriate amount of catalysts LAuCl and AgOTf, then add an appropriate amount of reaction solvent, stir at room temperature, an...
Embodiment 2
[0040] Embodiment 2, a kind of synthesis method of polysubstituted pyrrole derivatives, this synthesis method is represented by aromatic primary amine derivative shown in formula II, β-nitrostyrene and its derivatives shown in formula III, 1 shown in formula IV , 3-dicarbonyl compound as raw material, using LAuCl and AgOTf as catalysts, reacting at 50°C to generate polysubstituted pyrrole derivatives described in formula I; the specific steps: aromatic primary amine derivatives shown in formula II, formula III The molar ratio of β-nitrostyrene and its derivatives and 1,3-dicarbonyl compound shown in formula IV is 1.1:1.2:1, and L in LAuCl is a ligand, which is 2-(di-tert-butyl Phosphine)biphenyl or 2-dicyclohexylphosphine-2,4,6-triisopropylbiphenyl; the reaction solvent is ethyl acetate, acetonitrile or toluene. All the other are identical with embodiment 1.
Embodiment 3
[0041] Embodiment 3, a kind of synthesis method of polysubstituted pyrrole derivatives, this synthesis method is represented by aromatic primary amine derivatives shown in formula II, β-nitrostyrene and its derivatives shown in formula III, 1 shown in formula IV , 3-dicarbonyl compound as raw material, using LAuCl and AgOTf as catalysts, reacting at 30°C to generate polysubstituted pyrrole derivatives described in formula I; the specific steps: aromatic primary amine derivatives shown in formula II, formula III The molar ratio of the β-nitrostyrene and its derivatives and the 1,3-dicarbonyl compound shown in Formula IV is 0.9:1.1:1, and L in LAuCl is a ligand, which is benzotriazole; Described reaction solvent is dichloromethane or methyl tert-butyl ether; All the other are identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com