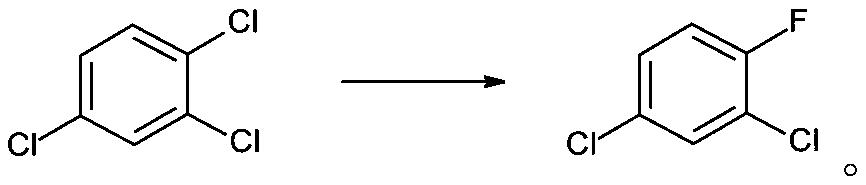
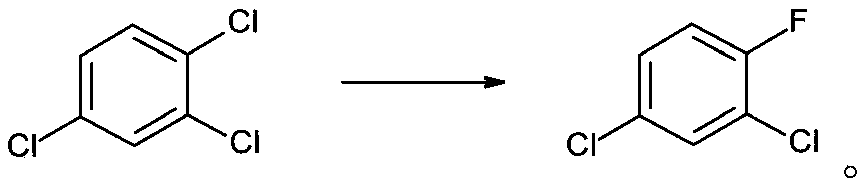
Synthesis method of 2, 4-dichlorofluorobenzene
A technology of dichlorofluorobenzene and a synthesis method, which is applied in the field of medicinal chemical synthesis, can solve the problems of high cost, high toxicity and high safety risk, and achieves the effects of short reaction route and safe synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0030] 5.74g of trimethylolpropane borate, 1.08g of p-tert-butylcalix[6]arene and 35.15g of KF were added to a solution of 100g of 1,2,4-trichlorobenzene dissolved in 150mL of sulfolane. The mixture was transferred into a 500mL pressure reactor, purged with nitrogen and replaced, sealed, heated to 185°C, and maintained at this temperature for 5h. The reactor was cooled to normal temperature, opened, and filtered to obtain 43.56 g of potassium salt; the filtrate was rectified under reduced pressure to obtain 83.66 g of 2,4-dichlorofluorobenzene with a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com