Method for preparing beta-nitrostyrolene and derivatives thereof
A technology of nitrostyrene and derivatives, which is applied in the fields of organic chemistry methods, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of small catalyst selection range, poor catalyst reuse, low catalyst activity, etc. The effect of good reusability, good industrialization prospect and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
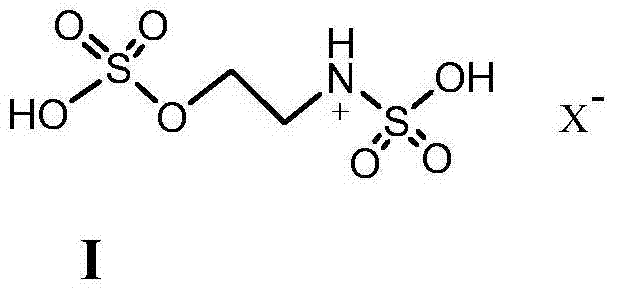
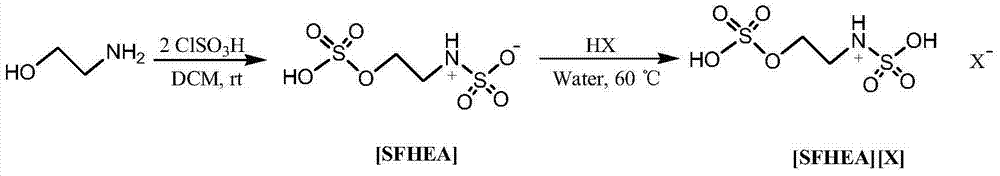
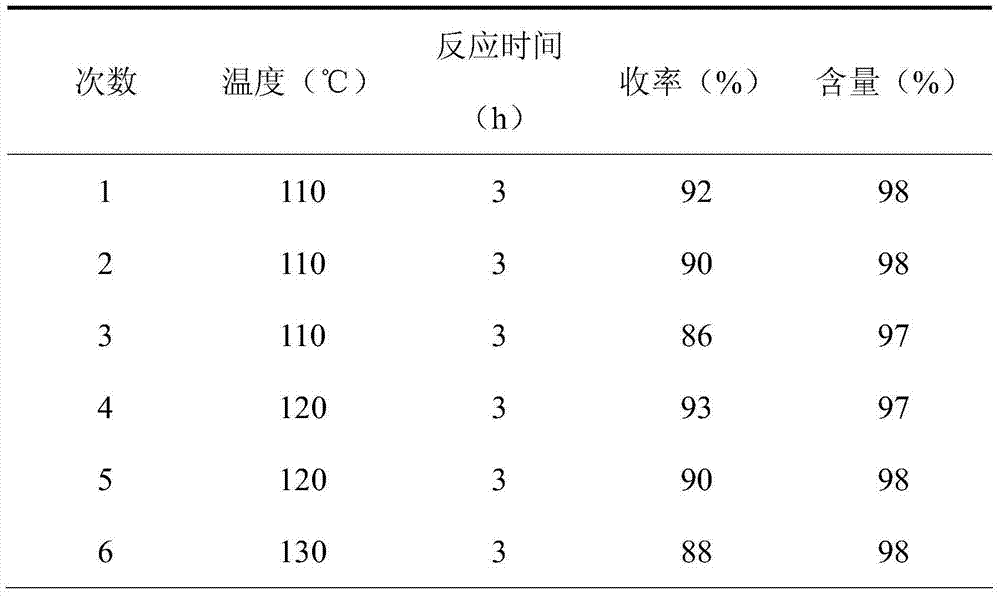
[0020] Benzaldehyde (5mmol), nitromethane (5.5mmol), 1mmol ionic liquid [SFHEA] [HSO 4 ] were sequentially added to a 50mL single-necked bottle, stirred at 110°C for 2 hours, detected by TLC, the raw materials disappeared, the reaction solution was extracted with ethyl acetate, the organic phase was combined, the solvent was evaporated under reduced pressure, and the product was obtained by recrystallization from absolute ethanol, with a yield of 91 %, the content is 98%.
[0021] β-nitrostyrene:
[0022] 1 H NMR (400MHz, CDCl 3 ):δ7.86(d,1H,J=2.8Hz),7.46-6.98(m,6H);ESI-MS:m / z=150[M+H].
Embodiment 2
[0024] P-methoxybenzaldehyde (5mmol), nitromethane (5mmol), 1mmol ionic liquid [SFHEA][HSO 4 ] was added to a 50mL single-necked bottle, stirred at 110°C for 3 hours, detected by TLC, the raw materials disappeared, the reaction solution was extracted with ethyl acetate, the organic phase was combined, the solvent was evaporated under reduced pressure, and the product was obtained by recrystallization from absolute ethanol, with a yield of 95% , content 98%.
[0025] 4-Methyoxy-trans-β-nitrostyrene:
[0026] 1 H NMR (400MHz, CDCl 3 ):δ7.96(d,1H,J=2.8Hz),7.54-7.50(m,3H),6.95(d,2H,J=7.2Hz),3.87(s,3H);ESI-MS:m / z=180[M+H].
Embodiment 3
[0028] P-methoxybenzaldehyde (5mmol), nitromethane (10mmol), 5mmol ionic liquid [SFHEA][HSO 4 ] was added to a 50mL single-necked bottle, stirred at 130°C for 3 hours, detected by TLC, the raw materials disappeared, the reaction solution was extracted with ethyl acetate, the organic phase was combined, the solvent was evaporated under reduced pressure, and the product was obtained by recrystallization from absolute ethanol, with a yield of 96% , content 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com