Preparation of efficient out-phase hydrogen bond donor MOF catalyst and application of catalyst
A technology for catalysts and catalytic reactions, applied in the direction of organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, organic chemistry, etc. Satisfaction and other problems, to achieve the effect of reducing self-polymerization and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Compound L 1 -H 2 preparation of
[0044]
[0045] (1) 3,5-bis(4-methoxycarbonylphenyl)benzene (L 1 -Me 2 )Synthesis
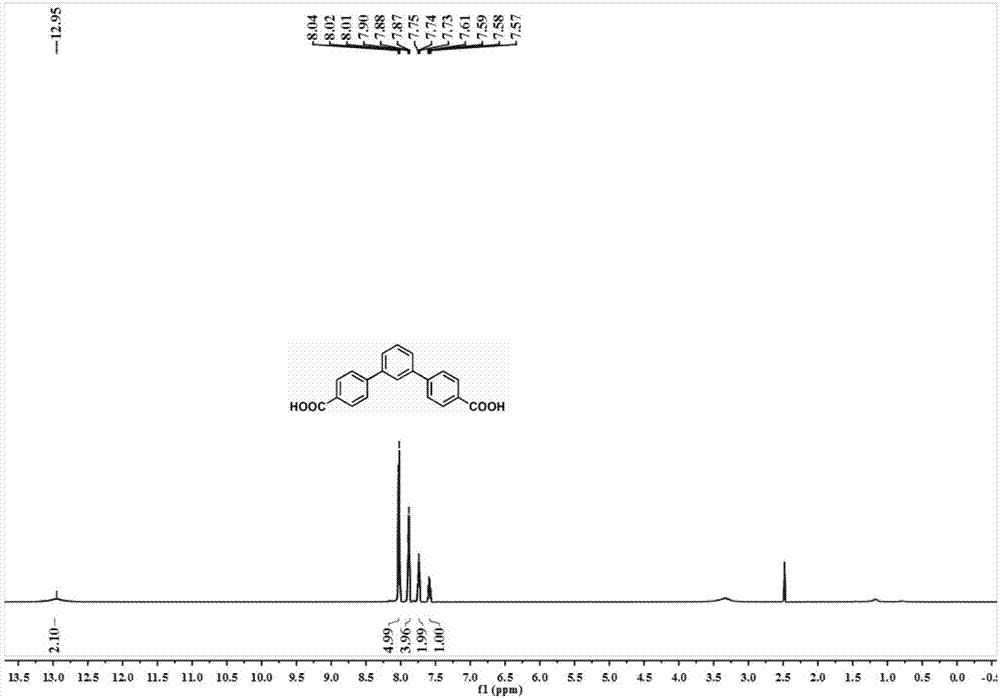
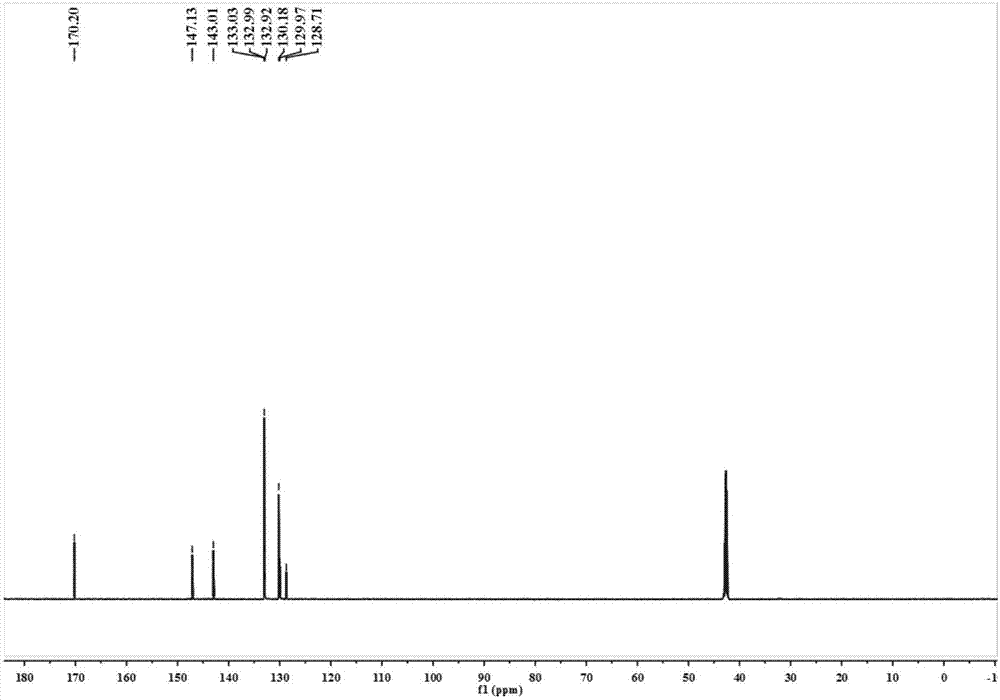
[0046] 1,3-Dibromobenzene (2.36g, 10mmol) was dissolved in a mixed solvent of THF and water (5:1, 120mL), and then 4-(methoxycarbonyl)phenylboronic acid (3.8 g, 25mmol), tetrakis(triphenylphosphine)palladium (0.232g, 0.2mmol) and sodium carbonate (3.18g, 30mmol). The resulting mixture was heated to reflux under nitrogen atmosphere for 20 hours. The reaction mixture was treated with H 2 O diluted and extracted with ethyl acetate. The obtained organic phase was washed with brine, washed with anhydrous Na 2 SO 4 dry. Remove Na 2 SO 4 After the solvent was evaporated, the residue was purified by silica gel column chromatography (hexane:ethyl acetate=20:1) to obtain 2.25 g of L 1 -Me 2 , yield 65%. 1 H NMR (400MHz, CDCl3) δ: 8.13 (d, J = 8.2Hz, 4H), 7.84 (s, 1H), 7.71 (d, J = 8.2Hz, 4H), 7.67-7.62 (m, 2H), 7.62 -7.51(m,1H),3.95(s...
Embodiment 2
[0049] Example 2 Compound L 2 -H 2 preparation of
[0050]
[0051] (1) N,N'-1-[3,5-bis(4-carboxymethylphenyl)phenyl]-3-phenylurea (L 2 -Me 2 )Synthesis
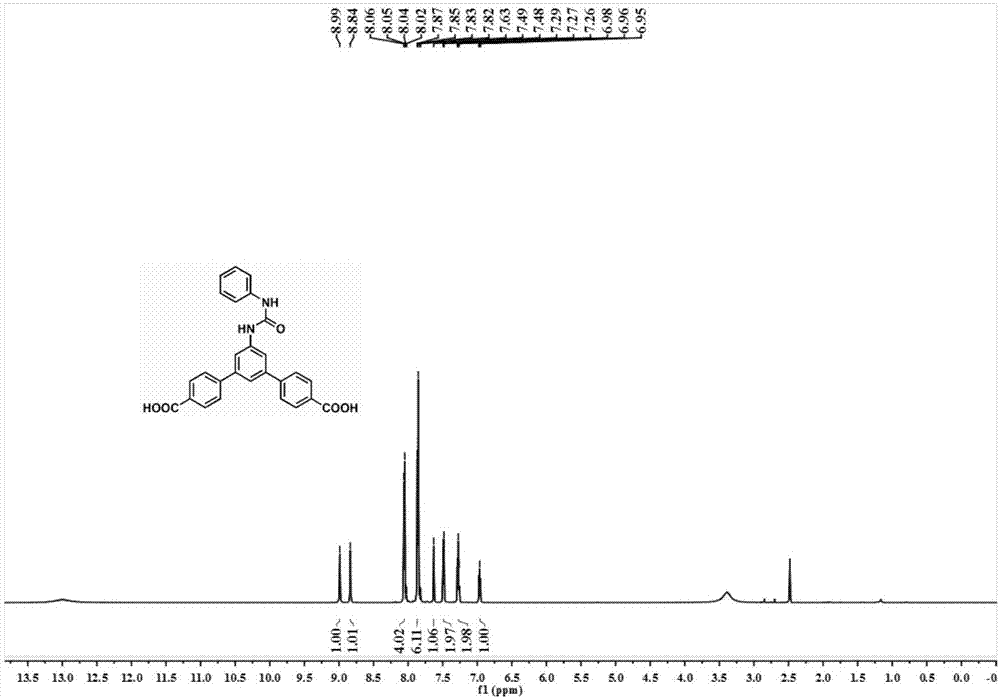
[0052] Benzoyl chloride (1.4 g, 10 mmol) was dissolved in 3 mL of dimethylformamide, then sodium azide (1.3 g, 20 mmol) was added. The solution was stirred at room temperature for 2 hours. The resulting reaction mixture was diluted with 30 mL of ethyl acetate. The organic phase was washed with brine, anhydrous Na 2 SO 4 dry. After removal of the solvent, the acyl azide was used without further purification. The crude acyl azide was diluted with anhydrous toluene (20 mL) under nitrogen, then heated to 80°C for 2 hours, and 3,5-bis(4-methoxycarbonylphenyl)aniline (2.77 g, 8mmol). The reaction was carried out at 80°C for 12 hours, and a white precipitate formed. The resulting precipitate was purified by chromatographic column, ethyl acetate: hexane = 1:5 eluent to obtain 3.2 g of product. 1 H NMR (400MHz, DMSO-d...
Embodiment 3
[0055] The preparation of embodiment 3 compound 1
[0056] will contain Cu(NO 3 ) 2 4H 2 O (5.18 mg, 0.02 mmol) and L 1 -H 2 (6.36mg, 0.02mmol) was put into a mixed solvent containing DMF (0.50mL), DMA (0.40mL) and water (0.10mL). The vial was sealed and fed at 80°C for 12 hours. The blue crystals were collected, washed with ethanol and acetone, and dried at room temperature. Obtained 7.0 mg, yield 65.0% (based on copper). IR(KBr):3006(w),2931(w),1942(w),1648(s),1598(s),1557(s),1513(m),1403(s),1261(m), 1188(m), 1103(m), 1016(m), 966(w), 906(w), 867(w), 801(w), 787(w), 771(s), 725(w), 706(w), 693(m), 652(w), 592(m), 519(s), 468(w), 416(w)cm -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com