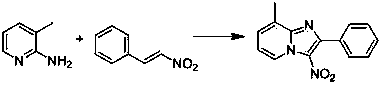Simple method for synthesizing imidazo (1,2-a) pyridine derivatives
A technology for synthesizing imidazole and 2-a, which is applied in organic chemistry and other fields, can solve problems such as unfavorable industrial production, high reaction temperature, and long reaction time, and achieve good regioselectivity, mild reaction conditions, and short process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: This embodiment is 3-nitro-2-phenyl-imidazo[1,2- a ] the synthesis of pyridine; With 2-aminopyridine and β-nitrostyrene as raw material, its reaction formula is as follows:
[0036]
[0037] Implementation steps:
[0038] 0.094 g (1 mmol) of 2-aminopyridine, 0.156 g (1.05 mmol) of β-nitrostyrene, 0.051 g (0.2 mmol) of iodine, 0.180 g (2 mmol) of tert-butanol peroxide and 0.049 g (0.62 mmol) of pyridine mmol) was placed in a reaction flask, 10 mL of methanol was added, heated to reflux (the temperature of the reaction solution was about 65° C.), and TLC was followed until the reaction was completed. After cooling to room temperature, methanol was removed by rotary evaporation, and the concentrated product was separated by chromatographic column to obtain the target product (yield 90%).
[0039] Product data: M.P. 172-174 ℃; 1 H NMR (400 MHz, CDCl 3 ) δ = 9.53 (d, J =6.9, 1H), 7.89 (dd, J=22.8, 5.7, 3H), 7.67 (t, J =7.9, 1H), 7.52 (d, J =2.6, ...
Embodiment 2
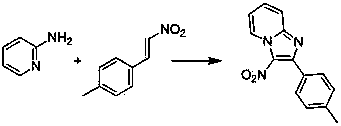
[0040] Embodiment 2: This embodiment is 3-nitro-2-(4-methylphenyl)-imidazo[1,2- a ] pyridine synthesis; with 2-aminopyridine and 4-methyl-β-nitrostyrene as raw materials, its reaction formula is as follows:
[0041]
[0042] Implementation steps:
[0043] 0.094 grams (1 mmol) of 2-aminopyridine, 0.171 grams (1.05 mmol) of 4-methyl-β-nitrostyrene, 0.051 grams (0.2 mmol) of iodine, 0.180 grams (2 mmol) of tert-butanol peroxide and Pyridine 0.049 g (0.62 mmol) was placed in a reaction flask, 10 mL of ethanol was added, heated to reflux (the temperature of the reaction solution was about 79° C.), followed by TLC until the reaction was completed. After cooling to room temperature, the ethanol was removed by rotary evaporation, and the concentrated product was separated by chromatographic column to obtain the target product (yield 87%).
[0044] Product data: M.P. 204-206 ℃; 1 H NMR (400 MHz, CDCl 3 ) δ = 9.51 (d, J =6.9, 1H), 7.83 (d, J =8.0, 3H), 7.64 (t, J =7.9, 1H...
Embodiment 3
[0045] Embodiment Three: This embodiment is 2-(4-methoxyphenyl)-3-nitro-imidazo[1,2- a ] The synthesis of pyridine; With 2-aminopyridine and 4-methoxy-β-nitrostyrene as raw material, its reaction formula is as follows:
[0046]
[0047] Implementation steps:
[0048] (1) 0.094 grams (1 mmol) of 2-aminopyridine, 0.283 grams (1.05 mmol) of 4-methoxy-β-nitrostyrene, 0.051 grams (0.2 mmol) of iodine, and 0.180 grams of tert-butanol peroxide ( 2 mmol) and 0.049 g (0.62 mmol) of pyridine were placed in a reaction flask, 10 mL of n-propanol was added, heated to reflux (the temperature of the reaction solution was about 97°C), and TLC was followed until the end of the reaction. After cooling to room temperature, propanol was removed by rotary evaporation, and the concentrated product was separated by chromatography column to obtain the target product (yield 85%).
[0049] Product Data: M.P. . 155-157℃; 1 H NMR (400 MHz, CDCl 3 ) δ 9.32 (d, J = 6.0 Hz, 1H), 7.94 (d, J =...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com