Patents
Literature
31 results about "Pyridine synthesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
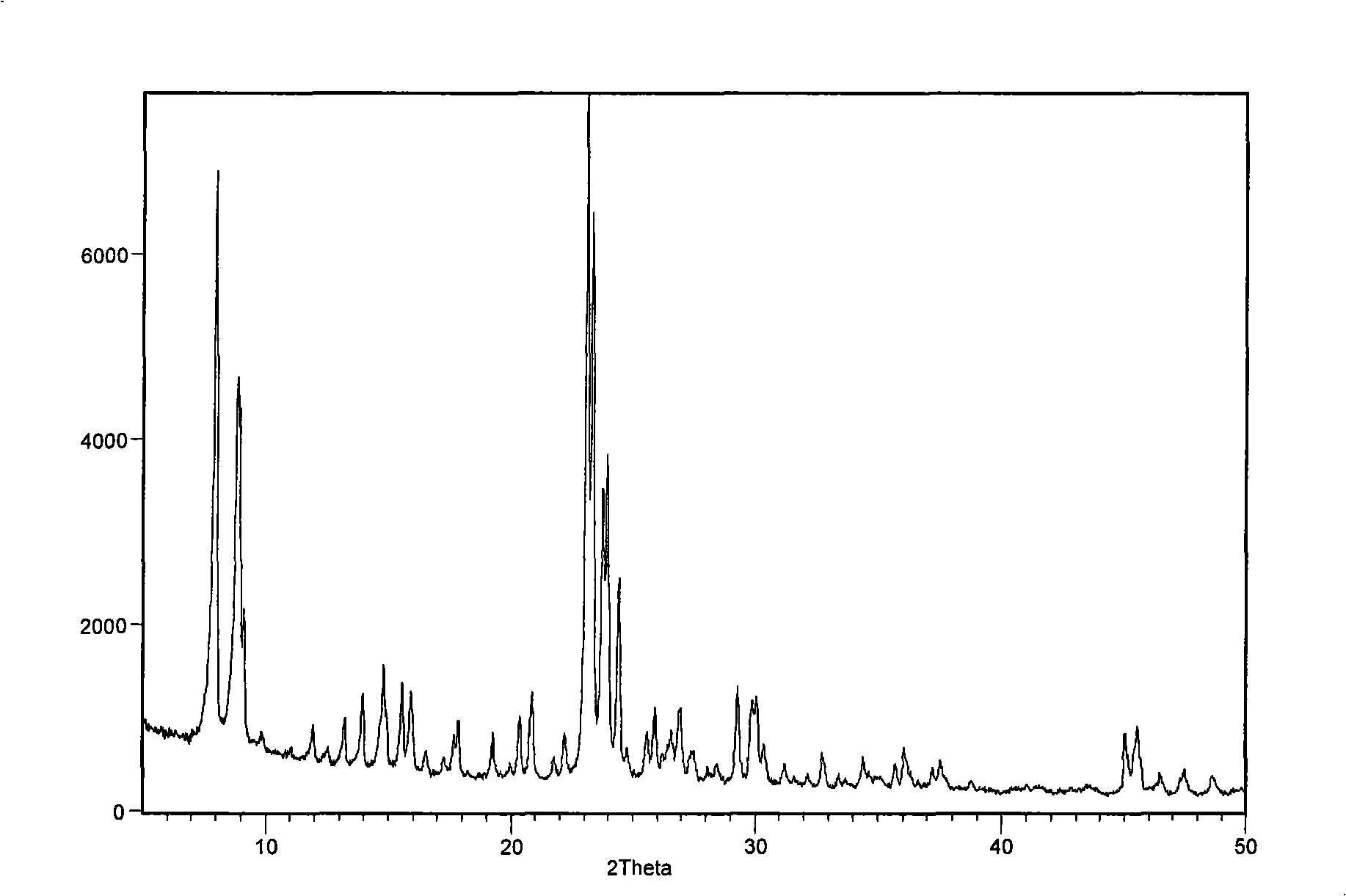
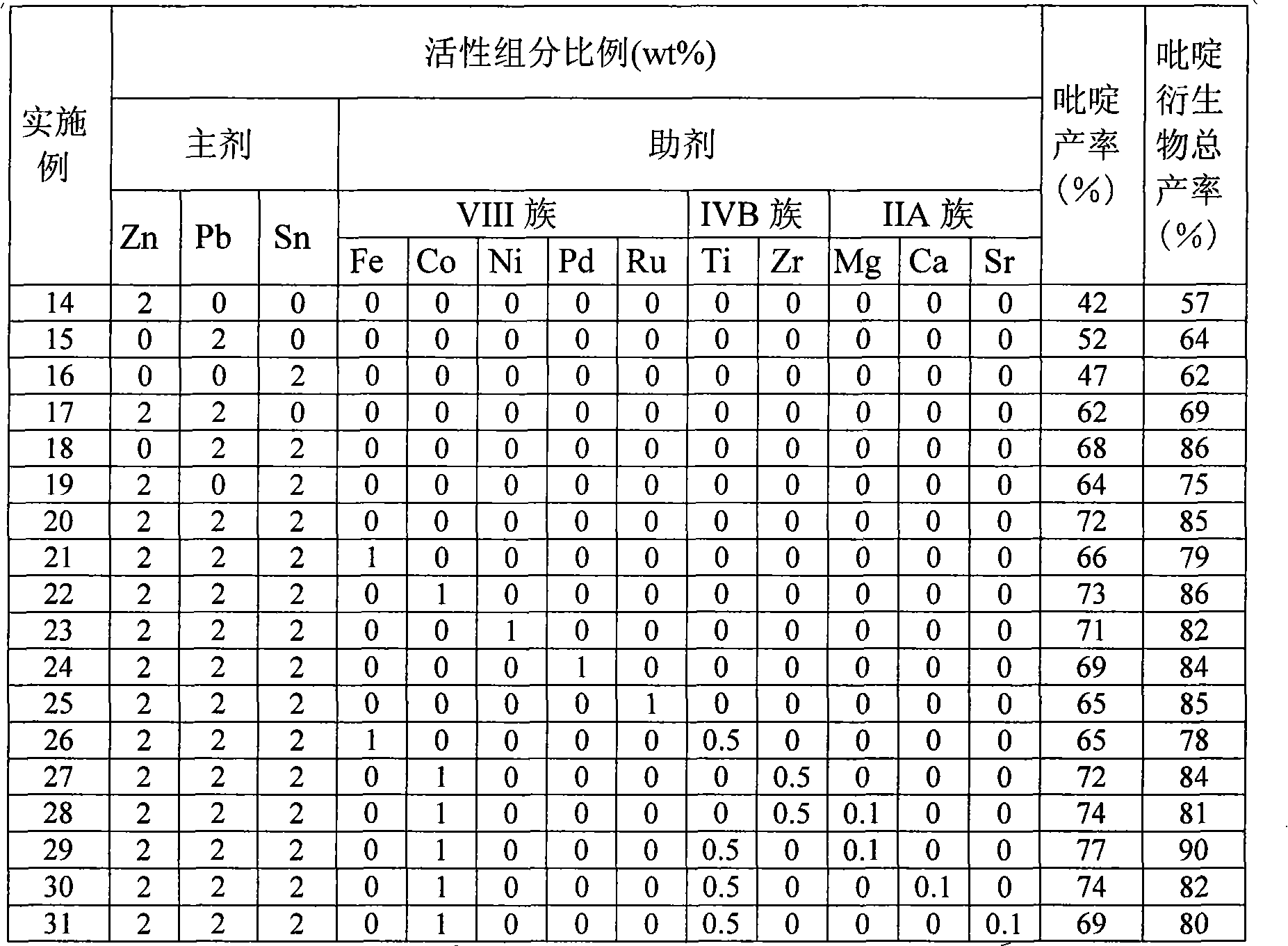
Catalyst for synthesizing pyridine using microsphere type high-silicon ZSM-5 molecular sieve as carrier and preparation method thereof
The invention provides a pyridine synthesis catalyst which takes microspherical high-silicon ZSM-5 molecular sieve as a carrier and a preparation method thereof. The preparation method adopts hydroxides of silicon source, aluminium source, alkali metal or alkaline earth metal, tetrapropylammonium bromide or tetrapropylammonium hydroxide and water as raw materials for preparing slurry, silicon-aluminum microspheres with the diameter of 30 to 200 um are obtained by spray drying and shaping, then the silicon-aluminum microspheres are arranged in organic amine steam, and the microspherical high-silicon ZSM-5 molecular sieve is obtained by washing, drying and calcination after the hydrothermal synthesis and the crystallization. The microspherical high-silicon ZSM-5 molecular sieve is taken as the carrier, one or more Pb, Sn and Zn elements is / are taken as main agents of active components, VIII family transition elements, IV family transition elements or / and alkaline earth metal elements are taken as auxiliary agents of the active components, wherein, the molar ratio of the total auxiliary agent to the total main agents is 0: 1 to 1: 1, the weight ratio of the active components and the carrier is 0.01 to 0.2; the carrier is impregnated in solution containing active components, and the catalyst of the invention is obtained by drying, calcination and activation, etc. As the synthesis process of the microspherical high-silicon ZSM-5 molecular sieve carrier which is used by the catalyst is shaped, the secondary shaping is unnecessary; at the same time, the catalyst can avoid the impact of a binding agent, thereby having better activity in applications. The yield of pyridine can achieve 77 percent and the total yield of pyridine derivatives can achieve 90 percent under the action of the catalyst. The catalyst of the invention is especially applicable to the fluidized bed catalytic process.
Owner:CHINA CATALYST HLDG CO LTD
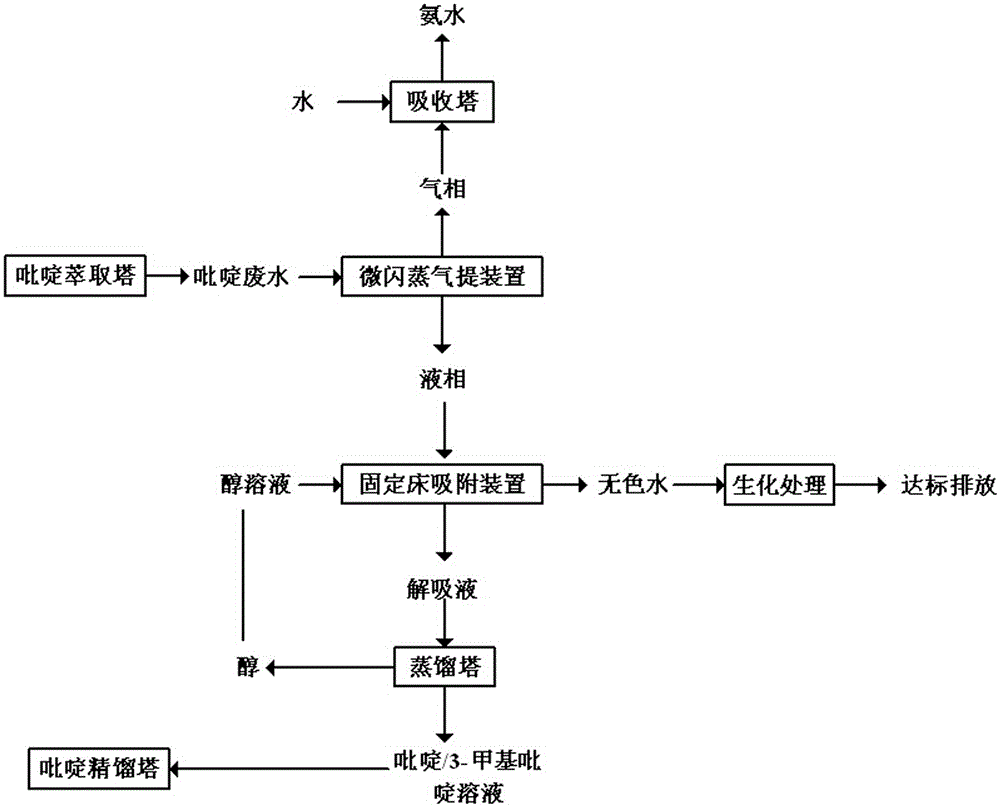
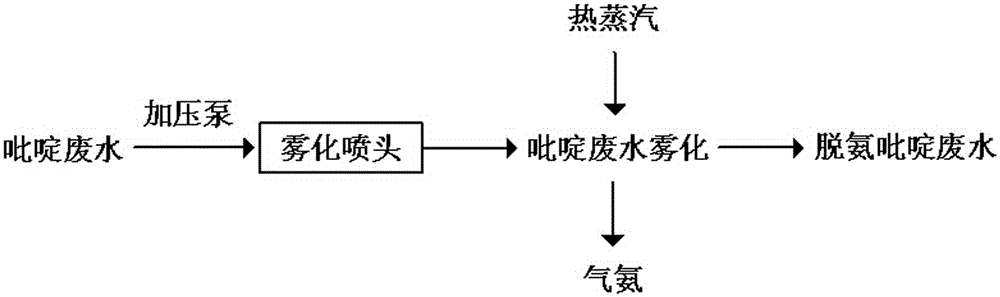
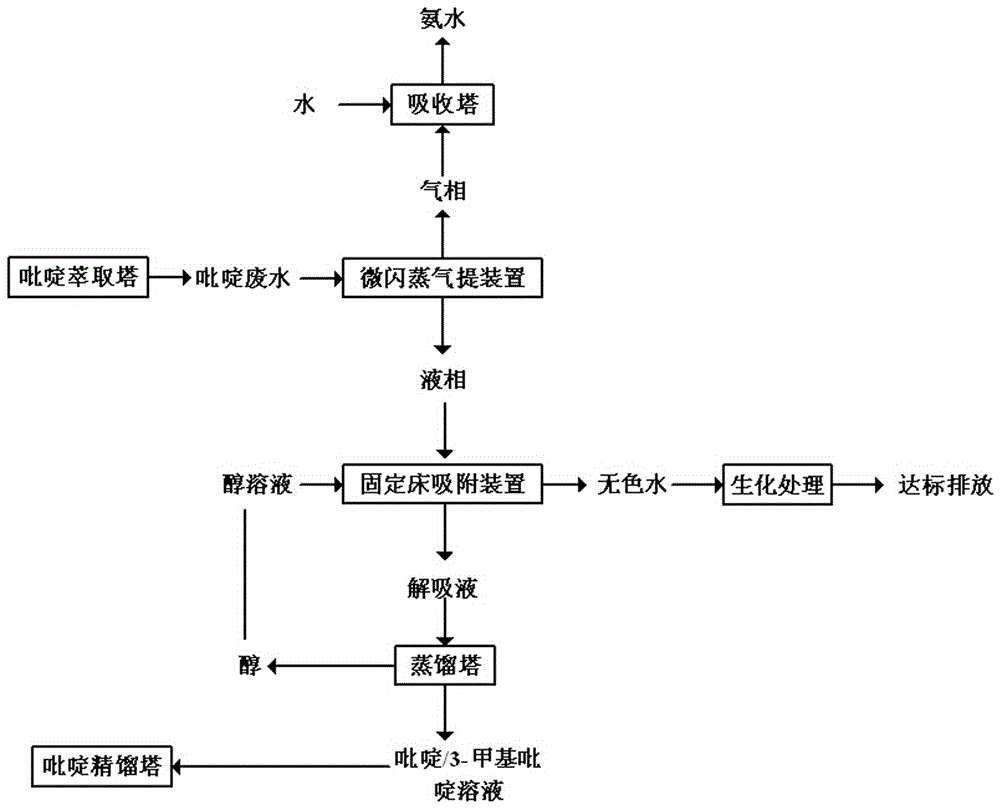
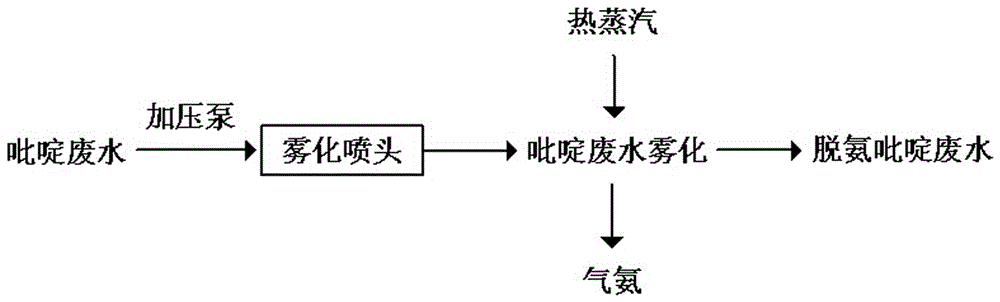
Method for treating pyridine wastewater
ActiveCN105084671AReduce processing costsOrganic chemistryMultistage water/sewage treatmentDesorptionDistillation
The invention discloses a method for treating pyridine wastewater, which comprises the following steps: pyridine wastewater, which is discharged from a pyridine extraction tower in an acetaldehyde+formaldehyde+liquid ammonia high-temperature pyridine synthesis technique, is subjected to low-vacuum flash distillation gas stripping, gas-phase absorption, fixed bed adsorption, desorption, distillation and other steps to separate and recover ammonia, pyridine and 3-methylpyridine in the pyridine wastewater, and the colorless water is subjected to biochemical treatment and discharged after reaching the standard. The method solves the problems of discharge of incinerator exhaust NOX, high incineration cost and carbon discharge due to abundant pyridine and derivatives in the wastewater incineration process in the pyridine production technique, effectively recovers the pyridine and derivatives, saves the cost, and lowers the pollution.
Owner:河北美邦工程科技股份有限公司
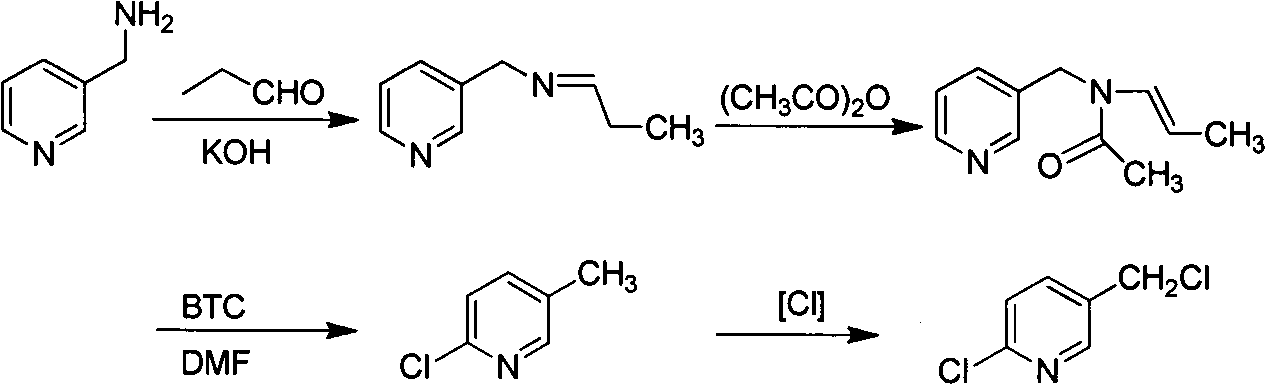
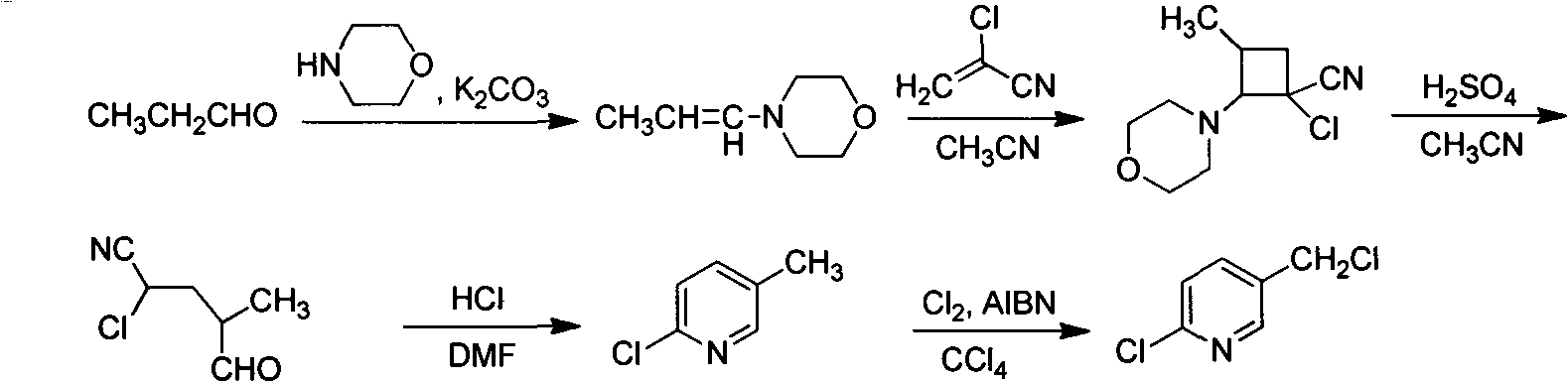
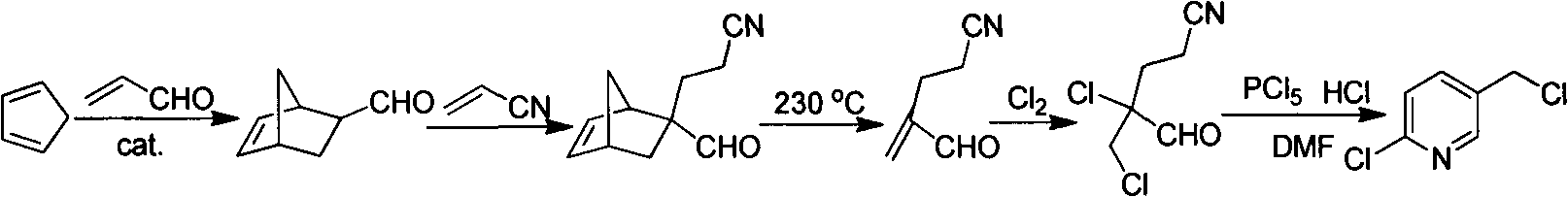
Improved 2-chlorine-5-chloromethyl pyridine synthesis process
InactiveCN102491943AAvoid disadvantagesRealize cleaner productionOrganic chemistryDistillationEthyl Chloride
The invention provides an improved 2-chlorine-5-chloromethyl pyridine synthesis process, which is characterized in that after reaction of toluene solution of 2-chlorine-2-chloromethyl-4-cyano butyraldehyde and triphosgene toluene solution is completed, a certain amount of trialkylamine is added in dropwise mode at 0 to 90 DEG C, trialkylamine hydrochloride is isolated by filtration, and filtrate is desolventized through distillation and then is decompressed and distilled to obtain products of the 2-chlorine-5-chloromethyl pyridine. The trialkylamine is one of triethylamine, tripropyl amine and tributylamine, quantity ratio of the triphosgene and the trialkylamine is 1:6.0 to 1:8.0, and the preferential ratio is 1:6.0 to 1:7.0. In the improved synthesis process, toluene obtained by distillation can be recycled, the main ingredients of a filter cake is the trialkylamine hydrochloride which can be sold as by-products after refining, and substrate after distillation is sent to a combustion furnace for treatment, thereby overcoming the shortcoming that the original technology generates a large amount of waste water, and achieving cleaner production of cyclization reaction.
Owner:NANJING UNIV OF TECH +2
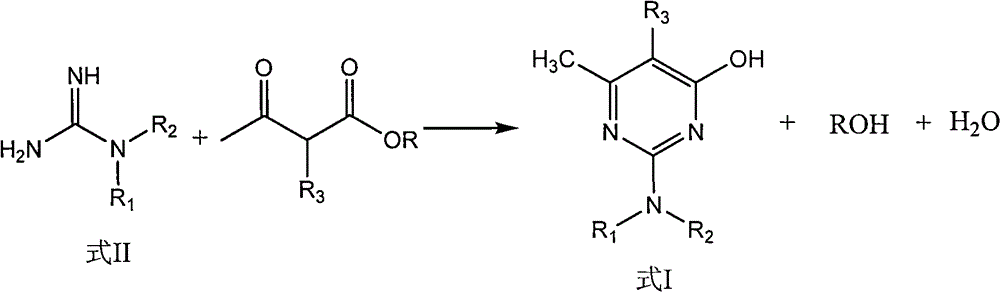
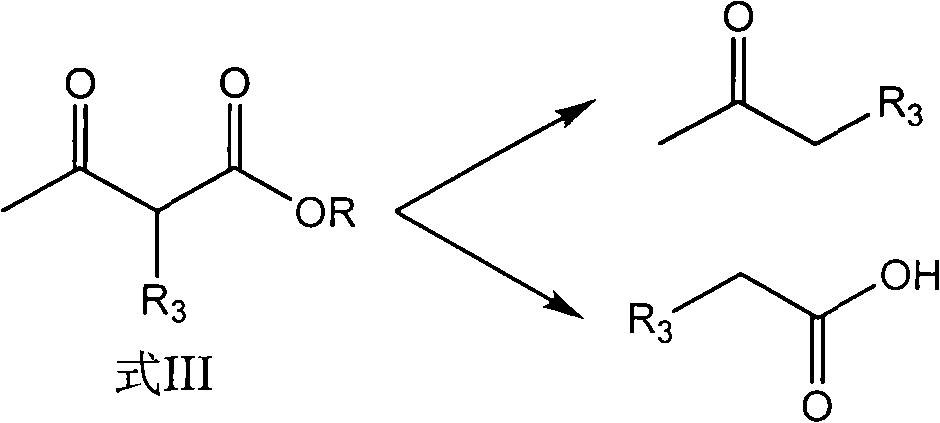
Preparation method of hydroxyl pyridine compound
The invention discloses a preparation method of a hydroxyl pyridine compound, which comprises the following steps of: taking hydrochloride or sulfate or nitrate of alkyl guanidine (shown in the formula II) as a reaction raw material; neutralizing with sodium hydroxide or potassium hydroxide; and then, taking toluene or xylene or chlorobenzene as a reaction solvent, and taking methanol or ethanol as a cosolvent for refluxing, dehydrating and dealcoholizing with alpha-alkyl acetoacetic ester (shown in the formula III) to react to obtain the hydroxyl pyridine compound (shown in the formula I). In the invention, by adding the methanol or ethanol as the cosolvent in the hydroxyl pyridine synthesis reaction, the solubility of the alkyl guanidine can be well increased, the decomposition of the reactants (alpha-alkyl acetoacetic ester and alkyl guanidine) is effectively controlled, the formation rate of the target product namely the hydroxyl pyridine compound shown in the general formula (I) is greatly increased, and the yield and content of the hydroxyl pyridine compound product shown in the general formula (I) are obviously improved, wherein the product yield is greater than 94%, and the product content is greater than 97%.
Owner:湖南海利常德农药化工有限公司
Supported catalyst for pyridine synthesis by tetrahydrofurfuryl alcohol and preparation process thereof
InactiveCN1736588AHigh selectivityHigh yieldOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsNitrateRoom temperature
The invention discloses a supported catalyst for synthesizing pyridine with tetrahydrofurfuryl alcohol and discloses the method for preparation, belonging to the catalyst technique for synthesizing pyridine. Said carrier of the catalyst is gamma- A1203, one of supporter components is MoO3, the mass of which is 5- 30% to that of carrier, another supporter component is V205, the mass of which is 5- 30% to that of carrier, and the third is La, Ce or Cd oxide, the mass of which is 0.5- 15% to that of carrier. The technique contains the following steps: Dissolving ammonium molybdate, ammonium metavanadate and a nitrate containing La, Ce or Cd into water to prepare an ammonium molybdate solution and a nitrate solution, mixing the dried gamma- A1203, mixing to make sure that the solution is uniformly absorbed into the micropore of the carrier aluminum oxide, drying in room temperature; redrying the room- temperature dried catalyst and igniting to prepare the supported catalyst that is used to synthesize pyridine with tetrahydrofurfuryl alcohol. The device needed is simple, the production is convenient, and the selectivity and yield of product pyridine is high.
Owner:TIANJIN UNIV
A method for treating pyridine wastewater
ActiveCN105084671BReduce processing costsOrganic chemistryMultistage water/sewage treatmentDistillationGas phase
Owner:河北美邦工程科技股份有限公司
Method for one-step direct preparation of 3-acyl imidazole [1, 5-a] pyridine through [4 +1] ketomethyl secondary amination reaction
The invention provides a method for one-step direct preparation of 3-acyl imidazole [1, 5-a] pyridine through [4 + 1] ketomethyl secondary amination reaction, and the method comprises the following steps: electrolyzing 1 equivalent of R1-ethyl ketone, 2 equivalent of pyridine-2-R2-formamide and 1.5 equivalent of NH4I for 16-20 hours in a diaphragm-free electrolytic bath under the constant current condition of 10-25 mA to obtain a product. The efficient electrochemical synthesis method of 3-acyl imidazole [1, 5-a] pyridine is developed, and the conventional multi-step reaction of synthesis of 3-acylimidazole [1, 5-a] pyridine is avoided through two times of amination of cheap ketone methyl sp3 hydrocarbon; the direct synthesis method, the cheap materials, the simple metal-free system, no additional electrolyte or oxidant and expandability enable the method to become a practical and green method for preparing 3-acyl imidazole [1, 5-a] pyridine and a large pi-system.
Owner:ANHUI SCI & TECH UNIV
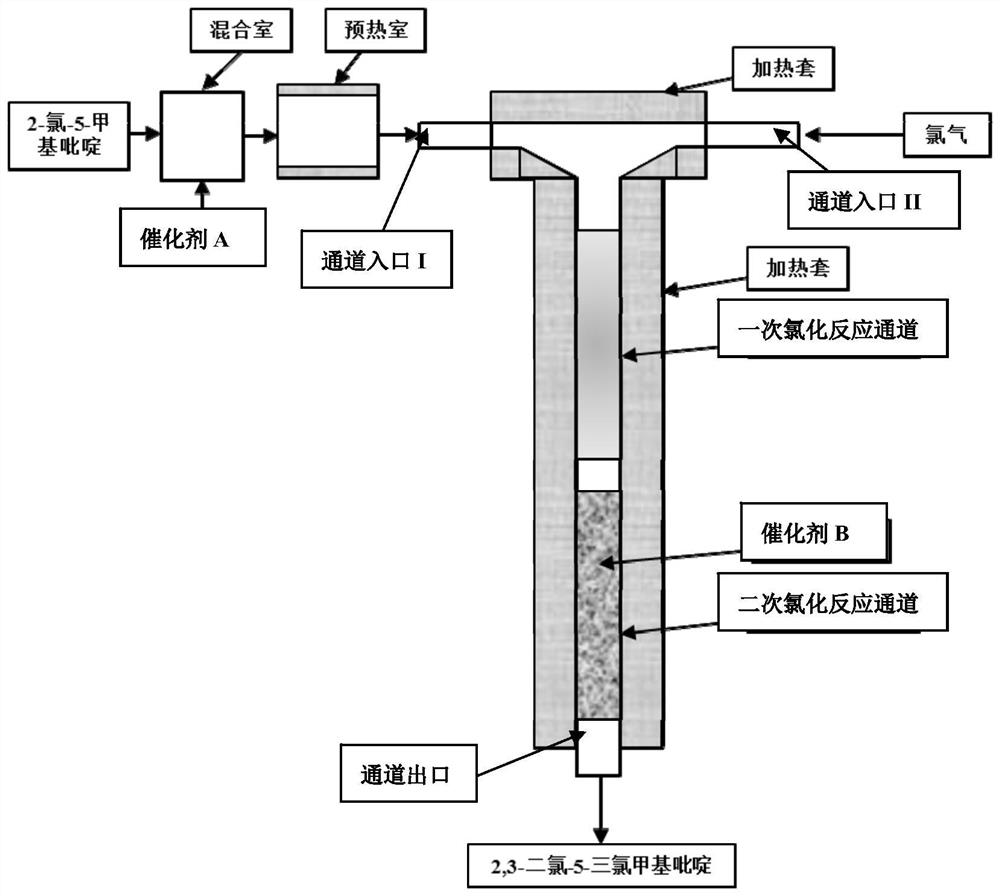
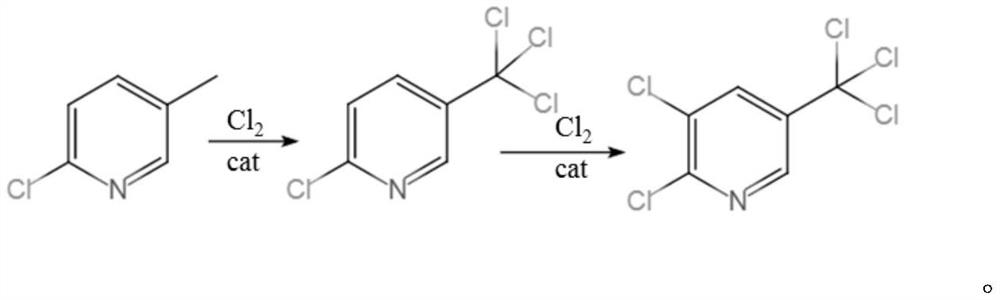
Synthesis method of 2, 3-dichloro-5-trichloromethylpyridine
InactiveCN112939853AHigh selectivityIncrease profitOrganic chemistryChemical/physical/physico-chemical microreactorsPtru catalystMeth-
The invention discloses a synthesis method of 2, 3-dichloro-5-trichloromethylpyridine, which is characterized in that a microreactor is used as a chlorination reaction channel, the first half of the microreactor channel is used as a primary chlorination channel, and the second half of the microreactor channel is filled with a catalyst B as a secondary chlorination reaction channel. The method comprises the following steps of firstly, fully mixing 2-chloro-5-methylpyridine with a catalyst A in a mixing chamber, then continuously reacting with chlorine through a primary chlorination channel to obtain 2-chloro-5-trichloromethylpyridine, then reacting through a secondary chlorination channel to obtain a crude oil layer of 2, 3-dichloro-5-trichloromethylpyridine, and carrying out alkali washing, dechlorination and rectification on the crude oil layer to obtain the high-concentration 2, 3 -dichloro-5-trichloromethylpyridine with the total yield to be 82-93%. According to the method, the technological process is simple, amplification production can be carried out through parallel connection, the catalyst B can be recycled and reused, the chlorination time is remarkably shortened, the chlorine utilization rate is increased, the post-treatment difficulty of the product is reduced, and the synthesis cost of 2, 3-dichloro-5-trichloromethylpyridine is saved.
Owner:JIANGSU YOUJIA CHEM
Preparation method of catalyst for combining pyridine
ActiveCN102039119ADoes not affect selectivityDoes not affect conversion rateOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsLead nitrateCerium
Owner:CHINA NAT OFFSHORE OIL CORP +1
1,4-dihydro-3,5-lauryl alcohol ester diacetate-2,6-dimethyl pyridine synthesis method
ActiveCN108299286AAvoid it happening againSolve instabilityOrganic chemistryAcetic acidHexamethylenetetramine
The invention discloses a 1,4-dihydro-3,5-lauryl alcohol ester diacetate-2,6-dimethyl pyridine synthesis method. The 1,4-dihydro-3,5-lauryl alcohol ester diacetate-2,6-dimethyl pyridine synthesis method comprises the following specific steps: 1, enabling lauryl alcohol and methyl acetoacetate to react to obtain n-dodecyl acetoacetate; 2, enabling the n-dodecyl acetoacetate to react with urotropinor formaldehyde and ammonium acetate to obtain the 1,4-dihydro-3,5-lauryl alcohol ester diacetate-2,6-dimethyl pyridine. The synthesis method uses the lauryl alcohol and the methyl acetoacetate to react to obtain the n-dodecyl acetoacetate, the production of amine wastewater is avoided compared with the reaction between the laurinol and ketene dimer, so that the synthesis method is a green production process, the problem of instability of the reaction material is also solved, and particularly, the synthesis method is higher in reaction yield and production purity and is suitable for industrialproduction.
Owner:CHANGZHOU YONGHE FINE CHEM
Novel method for synthesizing cyano-substituted imidazo[1,5-a]pyridine
InactiveCN113603687AEconomy of stepsLow costOrganic chemistryChemical synthesisCombinatorial chemistry
The invention discloses a novel method for synthesizing cyano-substituted imidazo[1,5-a]pyridine, and belongs to the field of chemical synthesis. The method comprises the steps: adding NH4SCN, pyridine-2-formaldehyde and amine into a dry reaction tube, and then adding a reaction solvent into the reaction tube; stirring the reaction system at room temperature for 5 minutes, adding I2O5, and further stirring and reacting under a heating condition; and after the reaction is finished, cooling a reaction mixture to room temperature, and quenching by using a saturated Na2S2O3 solution. The method provided by the invention is a synthesis method which is simple to operate, low in cost, efficient and green.
Owner:BEIJING UNIV OF TECH
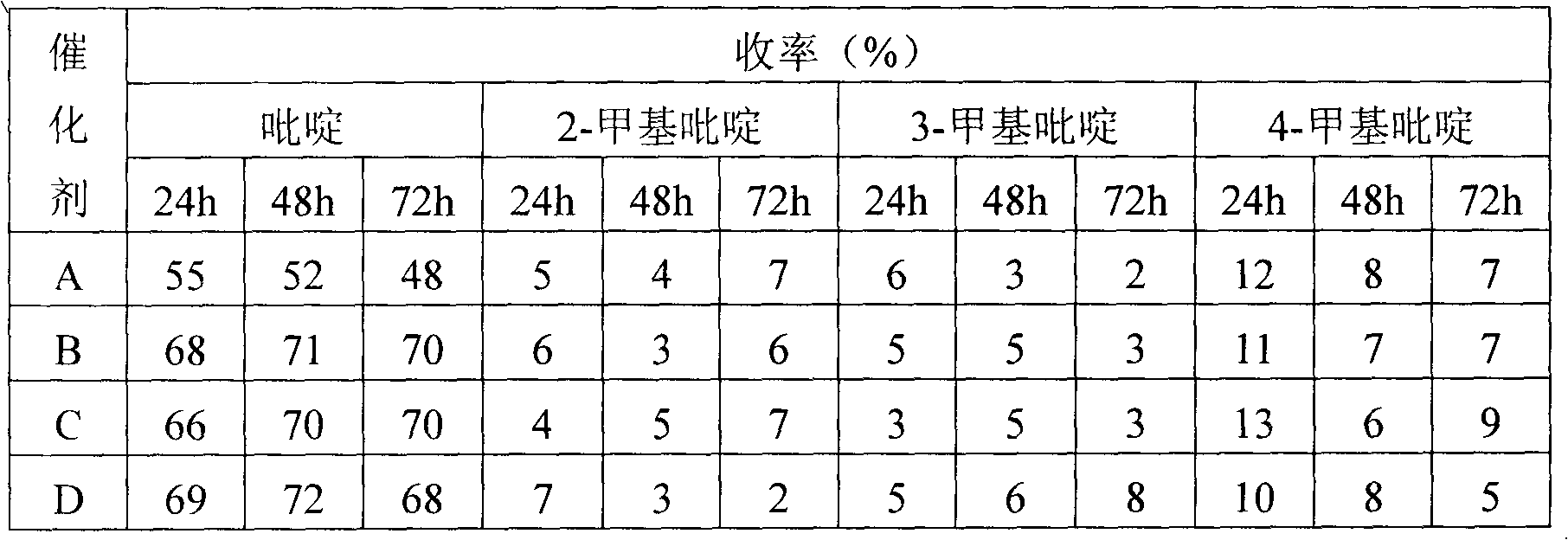
Pharmaceutical intermediate 2,6-dicarboxylic acid pyridine synthesis method
InactiveCN108239023AReduce intermediate linksShort reaction timeOrganic chemistryAntimony trichloridePyridine synthesis
The invention relates to a pharmaceutical intermediate 2,6-dicarboxylic acid pyridine synthesis method, which mainly comprises: adding 2 mol 2-isopropyl-3,5-dihydroxy-6-ethyl pyridine and 5-7 mol ethylene glycol-methyl ether solution into a reaction container, controlling the stirring speed at 190-230 rpm, increasing the temperature of the solution to 60-67 DEG C, adding 3-4 mol antimony trichloride, maintaining for 40-60 min, reducing the solution temperature to 5-9 DEG C, precipitating the precipitate, filtering, washing 3-7 times with a potassium nitrate solution, combining the filtrate andthe washing solution, controlling the stirring speed at 110-130 rpm, adjusting the pH value to 2-3 with an oxalic acid solution, washing with a N-benzylmethylamine solution, washing with a benzyl methyl ether solution, re-crystallizing in a propyl phenyl ketone solution, and precipitating the crystal to obtain the finished product 2,6-dicarboxylic acid pyridine.
Owner:CHENGDU DONG DIAN AI ER TECH
Synthesis method of 3, 4-dihydro-2H-pyrano [2, 3-b] pyridine
PendingCN112830933AEasy to separate and purifyReduce pollutionOrganic chemistryAcyl groupDouble bond
The invention relates to a synthesis method of 3, 4-dihydro-2H-pyrano [2, 3-b] pyridine. The problems of difficult availability of raw materials, expensive reagents, complicated operation, high risk, high energy consumption and the like in the existing route are mainly solved. The method is realized through the following technical scheme: (1) reacting 2-bromopyridine with a formylation reagent under the action of alkali to generate 2-bromo-3-formylpyridine; (2) reacting an aldehyde group of the 2-bromine-3-formylpyridine with triethyl phosphonoacetate to generate 2-bromine-3-pyridine ethyl acrylate; (3) simultaneously reducing double bonds and ester groups of 2-bromo-3-pyridine ethyl acrylate by lithium borohydride in one step while enabling bromine not to be influenced, so as to obtain 2-bromo-3-pyridine propanol; and (4) carrying out ring closing on the 2-bromo-3-pyridyl propanol under the action of alkali to obtain a target compound.
Owner:KANGHUA SHANGHAI DRUG RES DEV CO LTD
Catalyst for synthesizing pyridine using microsphere type high-silicon ZSM-5 molecular sieve as carrier and preparation method thereof
The invention provides a pyridine synthesis catalyst which takes microspherical high-silicon ZSM-5 molecular sieve as a carrier and a preparation method thereof. The preparation method adopts hydroxides of silicon source, aluminium source, alkali metal or alkaline earth metal, tetrapropylammonium bromide or tetrapropylammonium hydroxide and water as raw materials for preparing slurry, silicon-aluminum microspheres with the diameter of 30 to 200 um are obtained by spray drying and shaping, then the silicon-aluminum microspheres are arranged in organic amine steam, and the microspherical high-silicon ZSM-5 molecular sieve is obtained by washing, drying and calcination after the hydrothermal synthesis and the crystallization. The microspherical high-silicon ZSM-5 molecular sieve is taken as the carrier, one or more Pb, Sn and Zn elements is / are taken as main agents of active components, VIII family transition elements, IV family transition elements or / and alkaline earth metal elements are taken as auxiliary agents of the active components, wherein, the molar ratio of the total auxiliary agent to the total main agents is 0: 1 to 1: 1, the weight ratio of the active components and the carrier is 0.01 to 0.2; the carrier is impregnated in solution containing active components, and the catalyst of the invention is obtained by drying, calcination and activation, etc. As the synthesis process of the microspherical high-silicon ZSM-5 molecular sieve carrier which is used by the catalyst is shaped, the secondary shaping is unnecessary; at the same time, the catalyst can avoid the impact of a binding agent, thereby having better activity in applications. The yield of pyridine can achieve 77 percent and the total yield of pyridine derivatives can achieve 90 percent under the actionof the catalyst. The catalyst of the invention is especially applicable to the fluidized bed catalytic process.
Owner:CHINA CATALYST HLDG CO LTD
Novel method for synthesizing SBQ sensitizer 4-[2-(4-formyl phenyl )vinyl]pyridine
InactiveCN101503389BConform to the structureProof of feasibilityOrganic chemistryPhotosensitive materials for photomechanical apparatusBenzeneDistillation
Owner:ANHUI UNIVERSITY
A kind of cemetine analogue, its synthesis method and its application
ActiveCN109535157BSimple structureRaw materials are easy to getNervous disorderOrganic chemistryOrganic acidAlcohol
Owner:JIANGSU UNIV OF SCI & TECH
Preparation method of hydroxyl pyridine compound
The invention discloses a preparation method of a hydroxyl pyridine compound, which comprises the following steps of: taking hydrochloride or sulfate or nitrate of alkyl guanidine (shown in the formula II) as a reaction raw material; neutralizing with sodium hydroxide or potassium hydroxide; and then, taking toluene or xylene or chlorobenzene as a reaction solvent, and taking methanol or ethanol as a cosolvent for refluxing, dehydrating and dealcoholizing with alpha-alkyl acetoacetic ester (shown in the formula III) to react to obtain the hydroxyl pyridine compound (shown in the formula I). In the invention, by adding the methanol or ethanol as the cosolvent in the hydroxyl pyridine synthesis reaction, the solubility of the alkyl guanidine can be well increased, the decomposition of the reactants (alpha-alkyl acetoacetic ester and alkyl guanidine) is effectively controlled, the formation rate of the target product namely the hydroxyl pyridine compound shown in the general formula (I) is greatly increased, and the yield and content of the hydroxyl pyridine compound product shown in the general formula (I) are obviously improved, wherein the product yield is greater than 94%, and the product content is greater than 97%.
Owner:湖南海利常德农药化工有限公司
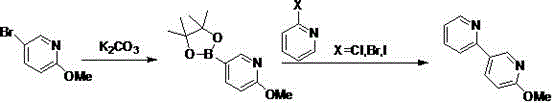
2-methoxy-5-(pyridine-2-yl)pyridine synthesis method
The invention belongs to the field of medicinal chemistry and relates to a preparation technology of 2-methoxy-5-(pyridine-2-yl)pyridine as a perampanel intermediate. The preparation technology comprises that 5-bromo-2-methoxypyridine and bis(pinacolato)diboron undergo a reaction to produce 2-methoxypyridine-5-boronic acid pinacol ester and the 2-methoxypyridine-5-boronic acid pinacol ester and 2-halogenated pyridine undergo a reaction to produce 2-methoxy-5-(pyridine-2-yl)pyridine as a perampanel intermediate. The synthesis method solves the problem of a high catalyst cost, has the advantages of simple processes and low cost and is convenient for large scale production.
Owner:BEIJING VENTUREPHARM BIOTECH
Synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester
InactiveCN112159423AShort stepsCheap and easy to getGroup 3/13 element organic compoundsBulk chemical productionDicarbonateCarboxylic acid
The invention discloses a synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester, and belongs to the field of medicinal chemistry. The synthesis method comprises the following steps: reacting 7-azaindole serving as a raw material with di-tert-butyl dicarbonate to synthesize 1H-pyrrolo[2,3b]pyridine-1-carboxylic acid tert-butyl ester, reacting the 1H-pyrrolo[2,3b]pyridine-1-carboxylic acid tert-butyl ester with n-butyl lithium and 1,2-dibromotetrafluoroethane at low temperature to synthesize 2-bromo-1H-pyrrolo[2,3-b]pyridine, synthesizing 2-bromo-1H-pyrrolo[2,3-b]pyridine-1-carboxylic acid tert-butyl ester, removing the protective group to obtain 2-bromo-1H-pyrrolo[2,3-b]pyridine, and reacting 2-bromo-1H-pyrrolo[2,3-b]pyridine with bi-pinacol borate under the catalytic action of transition metals to obtain the target product. The raw materials and reagents are cheap and easy to obtain, reaction conditions are mild, the safety is high, the cost is low, and the preparation method is easily applied to industrial batch production.
Owner:凯美克(上海)医药科技有限公司
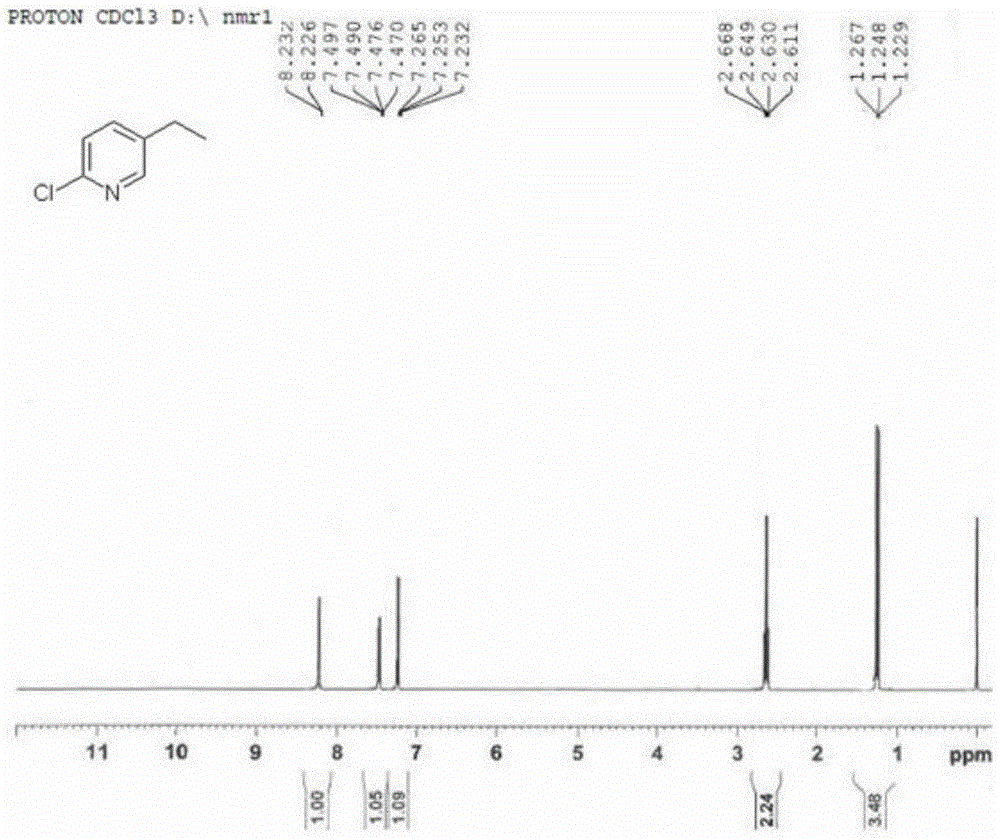
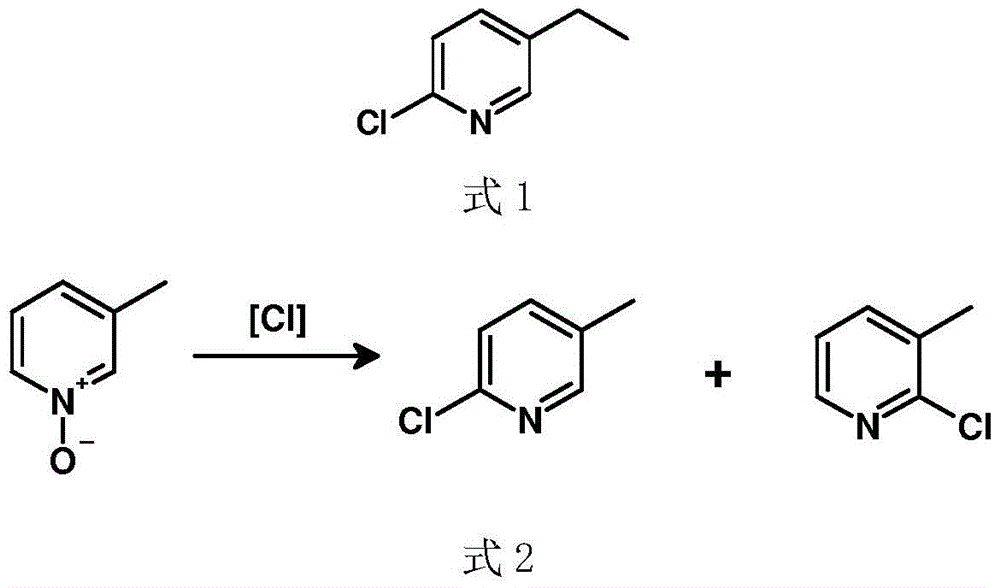
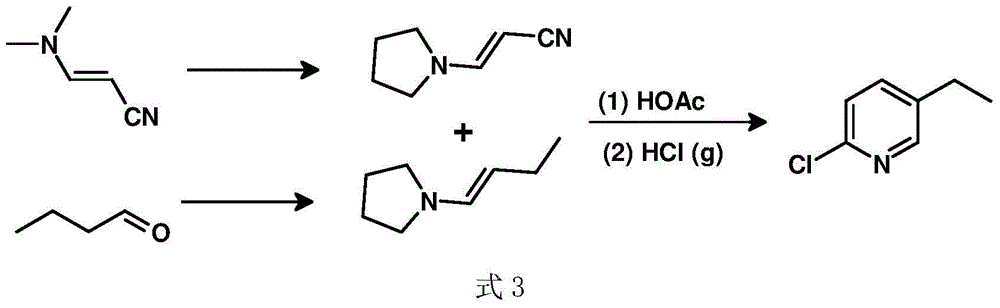
A kind of preparation method of 2-chloro-5-ethylpyridine
InactiveCN104529881BHigh selectivityHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsIridiumWittig reaction
Owner:SUZHOU SHIYA BIOPHARMLS
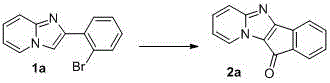
A kind of synthetic method of indenonoimidazopyridine compound
The invention discloses a method for synthesizing indene ketone and imidazole and pyridine compounds. The method comprises the steps that 2-(2- bromophenyl) imidazole [1, 2-alpha] pyridine or a derivative thereof is dissolved in solvent, then catalysts, ligands and alkali are added, a reaction is conducted in oil bath at the temperature of 120-160 DEG C under the atmosphere of 1atm of CO, and the 11H-indene [1', 2': 4, 5] imidazole [1, 2-alpha] pyridine-11-ketone compounds are obtained. The synthesis process is simple and efficient, a series-connection reaction under catalysis of transition metal is utilized, the indene ketone and imidazole and pyridine compounds are obtained through one step, in other words, an indene ketone structure is constructed while indanone and an imidazole and pyridine structural unit are linked together, resource waste and environmental pollution caused by application of multiple types of reagent andpurification treatment of the ligands in all steps of the reaction and the like in multiple steps of the reaction are avoided, raw materials are easy to prepare, the reaction is easy and convenient to operate, and the adaptability range of a substrate is wide.
Owner:HENAN NORMAL UNIV
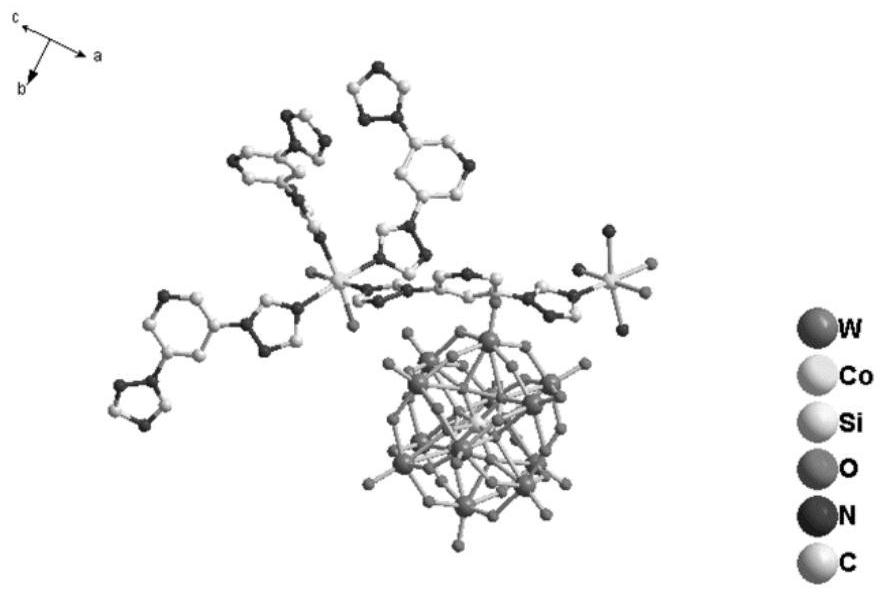
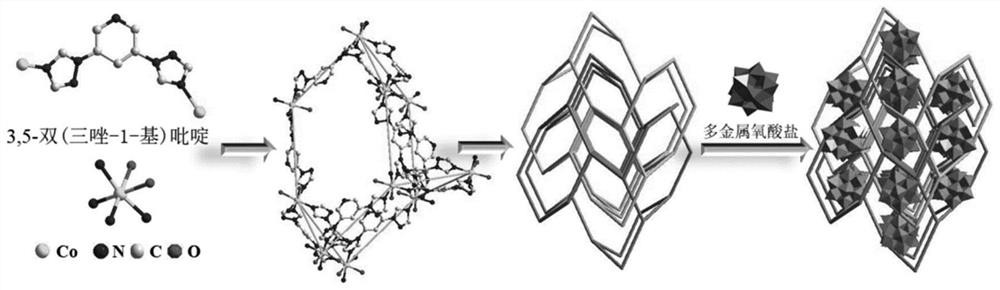
Preparation and catalytic performance of multi-acid-group cobalt organic framework with double interpenetrating structure
ActiveCN114805836AStable structureMaintain catalytic activityWater/sewage treatment by irradiationComponent separationPtru catalystMetal-organic framework
The invention relates to preparation and application of a multi-acid-group cobalt metal organic framework with a double interpenetrating structure. The invention aims to solve the problems of poor stability and low catalytic performance of some polyacid materials as catalyst materials in a solution, and provides a preparation method of a colorimetric sensing catalyst material and a photocatalytic degradation material capable of improving the catalytic performance of the polyacid materials. The chemical formula of the polyacid-based metal organic framework material with the double interpenetrating structure is [Co2 (btap) 4 (H2O) 4] [SiW12O40], and btap is 3, 5-bis (triazole-1-yl) pyridine. The synthesis method comprises the following steps: adding silicotungstic acid, cobalt acetate and btap into distilled water, uniformly stirring, adjusting the pH value, and reacting for 4 days at the temperature of 160 DEG C; the cobalt metal organic framework crystal material with the multi-acid-group double interpenetrating structure, which has colorimetric sensing and visible light catalysis performance, can be obtained.
Owner:HARBIN UNIV OF SCI & TECH
Tridentate [ONO] tungsten coordination compound and optical application thereof
PendingCN112851712ARedshift is obviousEnhanced glowLuminescent compositionsGroup 6/16 organic compounds without C-metal linkagesLight excitationToluene
The invention provides a tridentate [ONO] tungsten coordination compound and optical application thereof, and the tridentate [ONO] tungsten coordination compound is divided into but not limited to three structures, namely a structure I, a structure II and a structure III, the synthesis method of the tridentate tungsten coordination compound mainly comprises the following steps: mixing different equal amounts of tridentate [ONO] phenol imine compounds serving as ligands with dichlorotungsten dioxide and 2.2-3 times of pyridine, and carrying out reflux reaction at 115-135 DEG C for at least 24 hours by taking methylbenzene as a solvent, so as to obtain the tridentate [ONO] tungsten coordination compound. The tridentate [ONO] tungsten coordination compound is synthesized by using different tridentate [ONO] phenol imine compounds as ligands, tungsten dioxide dichloride and pyridine, the ligands are subjected to configuration torsion after optical excitation, and when metal tungsten is added to form a metal-ligand coordination bond, the configuration torsion of the ligands is limited, the energy relaxation is reduced, the ligand red shift is obvious, and the light is enhancedl; the tridentate [ONO] tungsten coordination compound can be used as a light-emitting material; and meanwhile, the synthesized complex has chirality, so that the synthesized complex can be used as a circular polarization luminescent material for practical application.
Owner:晨茵科创(天津)有限公司
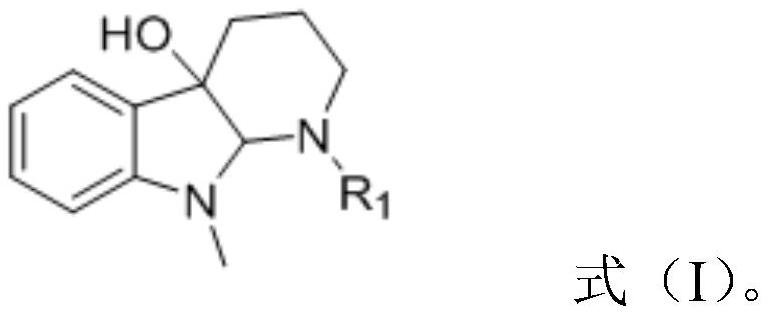
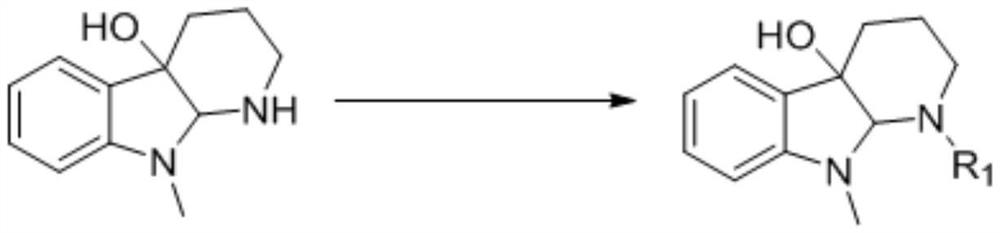
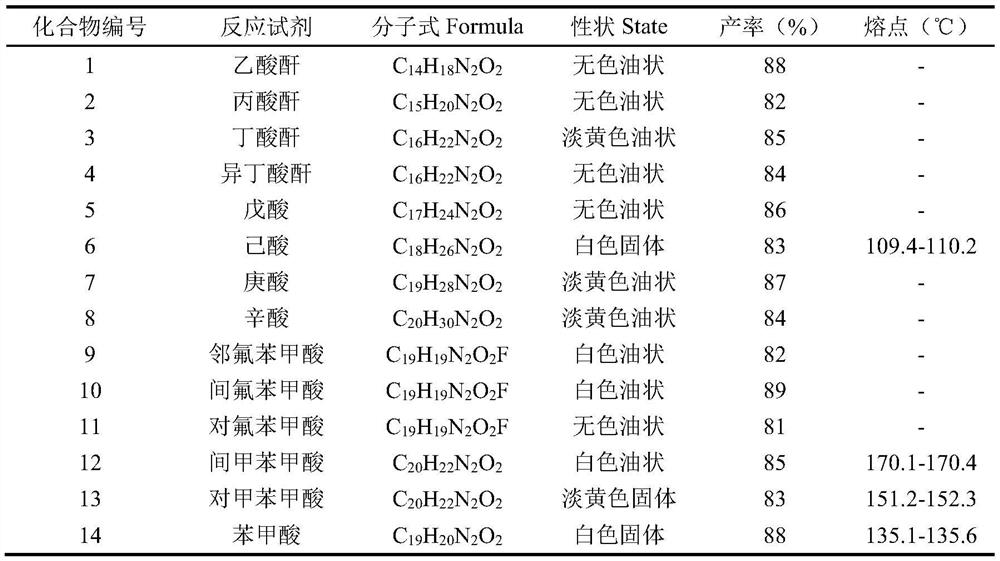
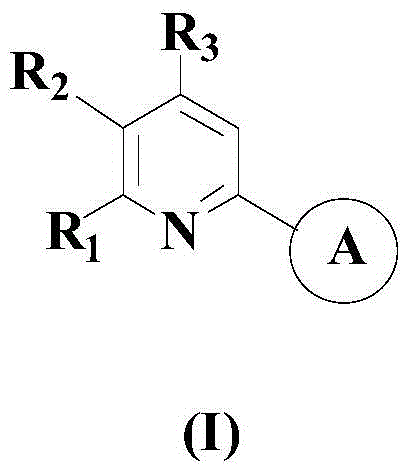
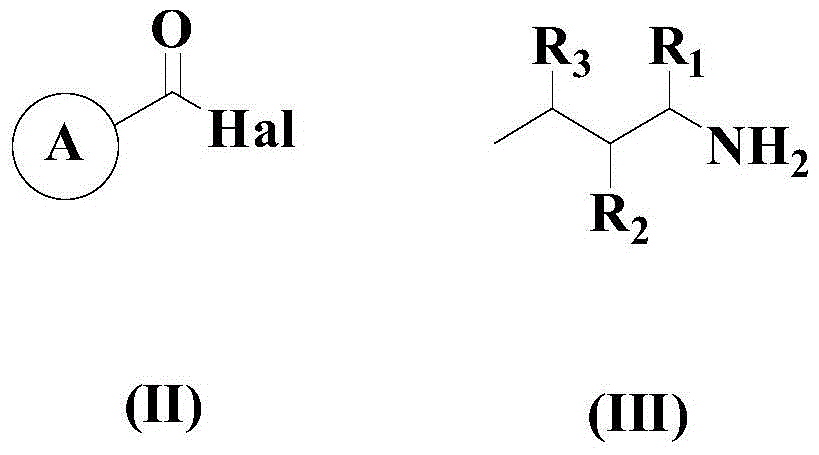
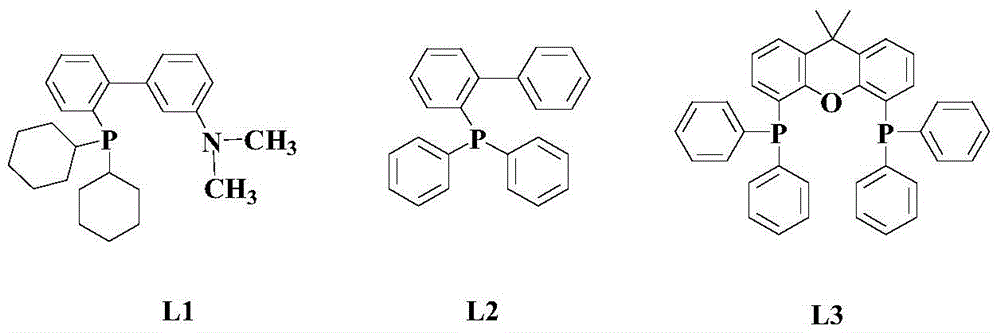
A kind of synthetic method of 2-substituted pyridine drug intermediate compound
The invention relates to a synthesis method of a 2-substituted pyridine-class drug intermediate compound shown in the formula (I) (please see the formula in the specification). The method includes the steps that when catalysts, ligand, alkali and additives exist, a compound with the formula (II) and a compound with the formula (III) react in an organic solvent so that the compound shown in the formula (I) (please see the formula in the specification) can be obtained, wherein R1, R2 and R3 are independently selected from H, halogen or C-C6 alkyl groups; the formula (please see the formula in the specification) is a C6-C10 aryl group which is not substituted or has 1-2 substitutent groups or is a C4-C10 heterocyclic radical which is not substituted or has 1-2 substitutent groups, and the substitutent grous are halogen, nitro or C1-C6 alkyl groups; Hal serves as the halogen. According to the method, the catalysts, the ligand, the alkali and the additives are properly selected and combined, and therefore a high yield is achieved, and the method has good industrial prospects and good application potentiality.
Owner:侯芳霖
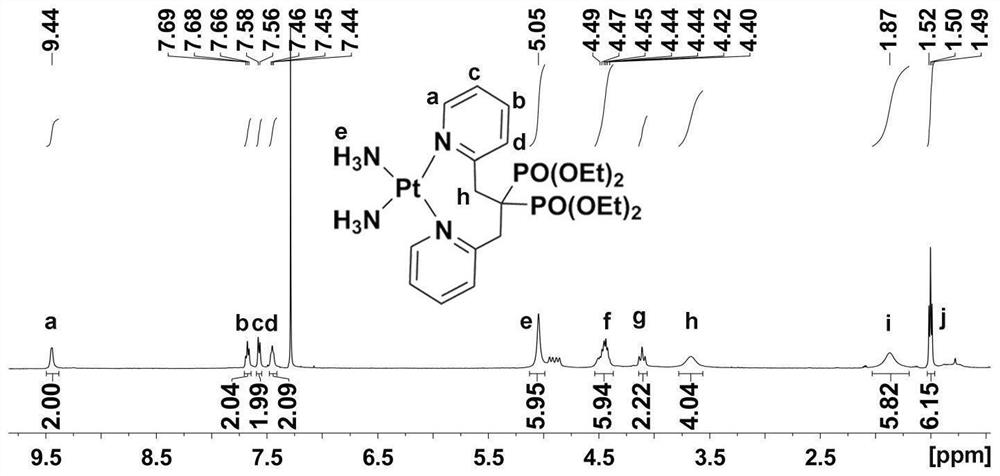
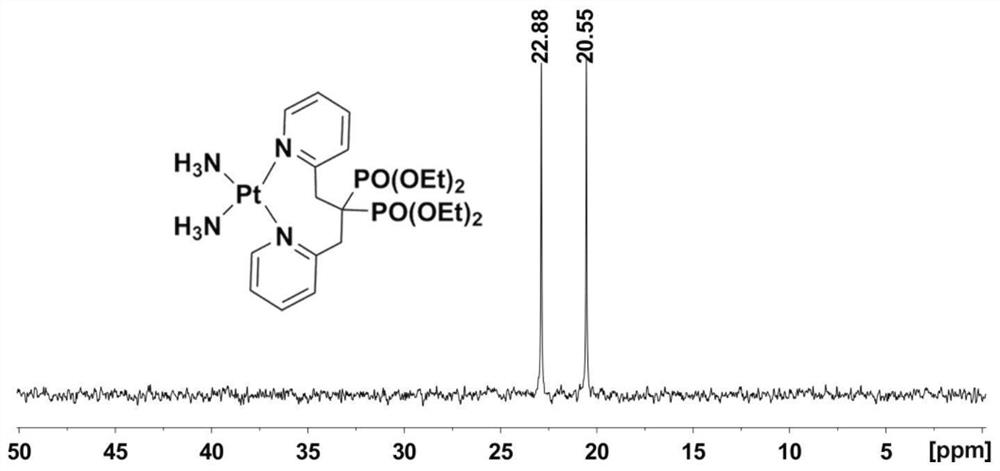
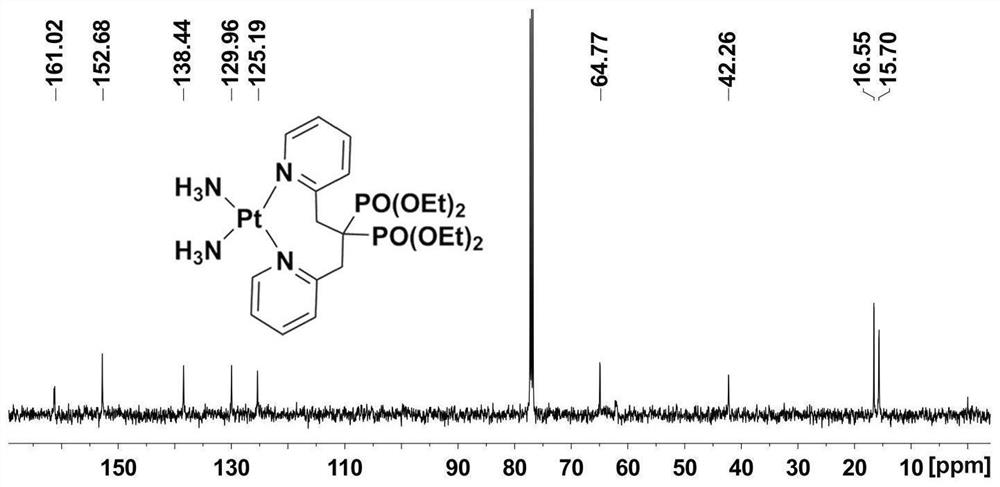
Platinum diphosphonate complex as well as synthesis method and application thereof
PendingCN114349794ANovel structureHigh synthetic yieldGroup 8/9/10/18 element organic compoundsAntineoplastic agentsLeaving groupGlutathione binding
The invention discloses a diphosphonic acid platinum complex and a preparation method thereof, a non-leaving group of the complex is composed of ammonia and a diphosphonic acid bipyridine ligand, and the diphosphonic acid bipyridine ligand and a platinum center form an eight-membered ring. The synthesis method of the complex comprises synthesis of 2-chloromethylpyridine, synthesis of [1, 3-bis (2-pyridyl) propane-2, 2-diyl] bis (phosphonic acid) tetraethyl ester and synthesis of the diphosphonic acid platinum complex, and the synthesis method is high in synthesis yield, high in implementation feasibility and stable in product. The complex is difficult to chelate with glutathione, so that side effects caused by combination of platinum drugs and glutathione can be greatly reduced, and the complex can be used for overcoming drug resistance of the platinum drugs; the complex has a certain inhibition effect on osteosarcoma cells; the complex can also interact with human serum albumin in a static quenching manner. The research provides a new strategy for solving the drug resistance of the platinum diphosphonate, and good technical progress is achieved.
Owner:NANJING MEDICAL UNIV
Preparation method of salazosulfapyridine composition
The invention provides a preparation method of a salazosulfapyridine composition. The preparation method of the salazosulfapyridine composition comprises the following steps: step 1, adding serine hydrazide hydrochloride and a catalyst into an organic solvent, and heating and stirring until the serine hydrazide hydrochloride and the catalyst are completely mixed to obtain a mixed solution; step 2,adding trihydroxybenzaldehyde into the mixed solution obtained in the step 1, and stirring until the trihydroxybenzaldehyde and the mixed solution are uniformly mixed to obtain a precursor solution;step 3, hydrogenating the precursor solution, adding a salazosulfapyridine crude body, mixing, evaporating to remove the organic solvent at a low temperature after hydrogenation is finished, and adding an extracting agent to extract crystals; and step 4, washing the crystal with ethanol or acetone to obtain the salazosulfapyridine composition. The preparation method of the salazosulfapyridine composition is simple in preparation process and suitable for popularization and application.
Owner:SUZHOU HUANGHE PHARMA CO LTD
Synthesis of nmicro-size and nanometer particles containing trigeminy ruthenium pyridine
InactiveCN100471929CGood electrochemiluminescent propertiesLuminescent compositionsPolyelectrolyteCapillary electrophoresis
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
A kind of method that manganese compound catalyzes oxidation pyridine compound to synthesize ketone
The invention discloses a method for synthesizing ketones or aldehydes by catalyzing the oxidation of pyridine compounds by manganese compounds. Pyridine compounds containing substituents are used as reaction substrates, manganese compounds are used as catalysts, and water, tert-butanol, acetonitrile, ethyl acetate or One or more than two kinds of dichloromethane as solvent, peroxide as oxygen source, react at 25-50°C for 12-48 h, so that the C-H bond of pyridine side chain is oxidized into ketone or aldehyde in one step, and the reaction The crude product was processed to obtain the final product. The preparation method of the invention has mild reaction conditions, less catalyst consumption, high atom economy, simple operation, wide application range of substrates and industrial applicability.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Supported catalyst for pyridine synthesis by tetrahydrofurfuryl alcohol and preparation process thereof
InactiveCN1319644CHigh selectivityHigh yieldOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsNitrateRoom temperature
The invention discloses a supported catalyst for synthesizing pyridine with tetrahydrofurfuryl alcohol and discloses the method for preparation, belonging to the catalyst technique for synthesizing pyridine. Said carrier of the catalyst is gamma- A1203, one of supporter components is MoO3, the mass of which is 5- 30% to that of carrier, another supporter component is V205, the mass of which is 5- 30% to that of carrier, and the third is La, Ce or Cd oxide, the mass of which is 0.5- 15% to that of carrier. The technique contains the following steps: Dissolving ammonium molybdate, ammonium metavanadate and a nitrate containing La, Ce or Cd into water to prepare an ammonium molybdate solution and a nitrate solution, mixing the dried gamma- A1203, mixing to make sure that the solution is uniformly absorbed into the micropore of the carrier aluminum oxide, drying in room temperature; redrying the room- temperature dried catalyst and igniting to prepare the supported catalyst that is used to synthesize pyridine with tetrahydrofurfuryl alcohol. The device needed is simple, the production is convenient, and the selectivity and yield of product pyridine is high.
Owner:TIANJIN UNIV
Synthetic process of 5-bromo-3-methyl-1h-pyrazolo[3,4-b]pyridine
The invention relates to a synthesizing process of 5-bromo-3-methyl-1H-pyrazolo [3, 4-B] pyridine. The synthesizing process comprises the following steps: 5-bromine-3-acetenyl-2-chlorine-pyridine is dissolved into DMF, then hydrazine, calcium carbonate and aluminium silicate are added sequentially and mixed uniformly, the mixture is reacted for 24-36 hours at the temperature of 1 DEGC to 10 DEG C, insoluble substances are filtered, the pressure is reduced to steam a solvent, and ethyl alcohol is used for recrystallization, so that the 5-bromo-3-methyl-1H-pyrazolo [3, 4-B] pyridine can be obtained. According to the synthesizing process, the aluminium silicate is used as a catalyst, so that the operation steps are simple, the yield is higher and industrial large-scale production of the 5-bromo-3-methyl-1H-pyrazolo [3, 4-B] pyridine is facilitated.
Owner:上海泰坦科技股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for one-step direct preparation of 3-acyl imidazole [1, 5-a] pyridine through [4 +1] ketomethyl secondary amination reaction Method for one-step direct preparation of 3-acyl imidazole [1, 5-a] pyridine through [4 +1] ketomethyl secondary amination reaction](https://images-eureka.patsnap.com/patent_img/2e1f949c-a4a3-4217-b9e6-a24db97ec781/211214093404.png)
![Method for one-step direct preparation of 3-acyl imidazole [1, 5-a] pyridine through [4 +1] ketomethyl secondary amination reaction Method for one-step direct preparation of 3-acyl imidazole [1, 5-a] pyridine through [4 +1] ketomethyl secondary amination reaction](https://images-eureka.patsnap.com/patent_img/2e1f949c-a4a3-4217-b9e6-a24db97ec781/211214093407.png)
![Method for one-step direct preparation of 3-acyl imidazole [1, 5-a] pyridine through [4 +1] ketomethyl secondary amination reaction Method for one-step direct preparation of 3-acyl imidazole [1, 5-a] pyridine through [4 +1] ketomethyl secondary amination reaction](https://images-eureka.patsnap.com/patent_img/2e1f949c-a4a3-4217-b9e6-a24db97ec781/211214093411.png)
![Novel method for synthesizing cyano-substituted imidazo[1,5-a]pyridine Novel method for synthesizing cyano-substituted imidazo[1,5-a]pyridine](https://images-eureka.patsnap.com/patent_img/0671e5ec-4f45-4424-b296-d5e3eadd9b2a/FDA0003192801100000011.png)
![Novel method for synthesizing cyano-substituted imidazo[1,5-a]pyridine Novel method for synthesizing cyano-substituted imidazo[1,5-a]pyridine](https://images-eureka.patsnap.com/patent_img/0671e5ec-4f45-4424-b296-d5e3eadd9b2a/BDA0003192801110000021.png)
![Novel method for synthesizing cyano-substituted imidazo[1,5-a]pyridine Novel method for synthesizing cyano-substituted imidazo[1,5-a]pyridine](https://images-eureka.patsnap.com/patent_img/0671e5ec-4f45-4424-b296-d5e3eadd9b2a/BDA0003192801110000022.png)
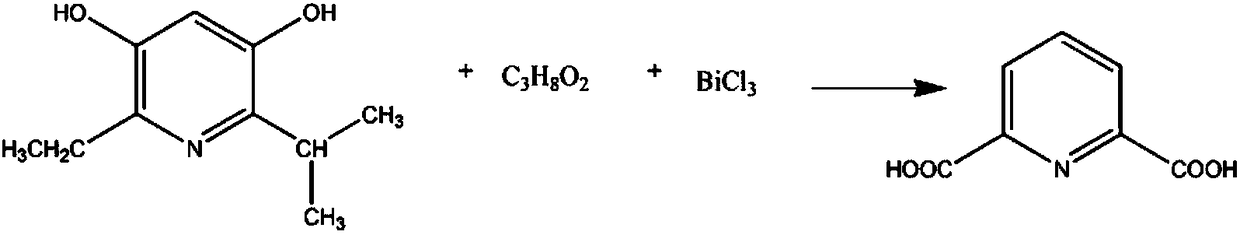
![Synthesis method of 3, 4-dihydro-2H-pyrano [2, 3-b] pyridine Synthesis method of 3, 4-dihydro-2H-pyrano [2, 3-b] pyridine](https://images-eureka.patsnap.com/patent_img/0fd6514c-d7f6-440f-b8d0-7e5c464a94db/DEST_PATH_IMAGE003.png)

![Novel method for synthesizing SBQ sensitizer 4-[2-(4-formyl phenyl )vinyl]pyridine Novel method for synthesizing SBQ sensitizer 4-[2-(4-formyl phenyl )vinyl]pyridine](https://images-eureka.patsnap.com/patent_img/2561d472-9e2d-4414-af4b-9b29b515269a/H2008102430988E00011.png)
![Novel method for synthesizing SBQ sensitizer 4-[2-(4-formyl phenyl )vinyl]pyridine Novel method for synthesizing SBQ sensitizer 4-[2-(4-formyl phenyl )vinyl]pyridine](https://images-eureka.patsnap.com/patent_img/2561d472-9e2d-4414-af4b-9b29b515269a/H2008102430988E00012.png)
![Novel method for synthesizing SBQ sensitizer 4-[2-(4-formyl phenyl )vinyl]pyridine Novel method for synthesizing SBQ sensitizer 4-[2-(4-formyl phenyl )vinyl]pyridine](https://images-eureka.patsnap.com/patent_img/2561d472-9e2d-4414-af4b-9b29b515269a/H2008102430988E00013.png)
![Synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester Synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester](https://images-eureka.patsnap.com/patent_img/424e69f3-6d8b-4524-b5fc-9b76cda70c43/HDA0002758107320000011.png)
![Synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester Synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester](https://images-eureka.patsnap.com/patent_img/424e69f3-6d8b-4524-b5fc-9b76cda70c43/HDA0002758107320000012.png)
![Synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester Synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester](https://images-eureka.patsnap.com/patent_img/424e69f3-6d8b-4524-b5fc-9b76cda70c43/FDA0002758107300000011.png)
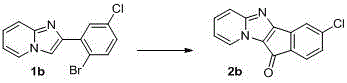
![Tridentate [ONO] tungsten coordination compound and optical application thereof Tridentate [ONO] tungsten coordination compound and optical application thereof](https://images-eureka.patsnap.com/patent_img/74ab16dd-dcc3-43cc-a8c9-226cdbd795c7/HDA0002868621920000011.png)
![Tridentate [ONO] tungsten coordination compound and optical application thereof Tridentate [ONO] tungsten coordination compound and optical application thereof](https://images-eureka.patsnap.com/patent_img/74ab16dd-dcc3-43cc-a8c9-226cdbd795c7/HDA0002868621920000012.png)
![Tridentate [ONO] tungsten coordination compound and optical application thereof Tridentate [ONO] tungsten coordination compound and optical application thereof](https://images-eureka.patsnap.com/patent_img/74ab16dd-dcc3-43cc-a8c9-226cdbd795c7/HDA0002868621920000013.png)

![Synthetic process of 5-bromo-3-methyl-1h-pyrazolo[3,4-b]pyridine Synthetic process of 5-bromo-3-methyl-1h-pyrazolo[3,4-b]pyridine](https://images-eureka.patsnap.com/patent_img/f519e9e1-3c4c-4d9e-b5d0-c99b8bb9173a/BDA0000809876250000021.png)