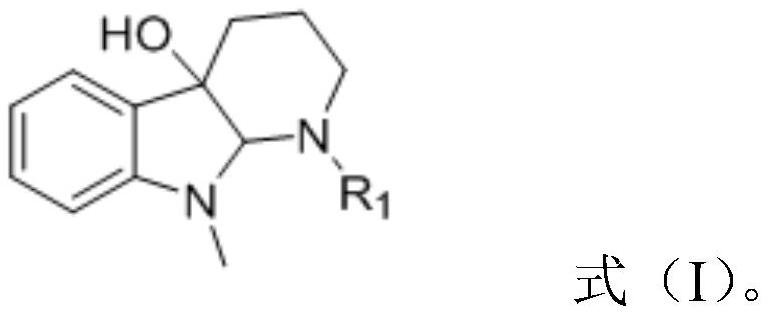
A kind of cemetine analogue, its synthesis method and its application
A technology of wax plum and its analogues, applied in the field of drug synthesis, can solve the problems of no effective prevention and treatment methods, and affect the health of the elderly, and achieve the effects of excellent application prospects, simple structure, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Synthesis
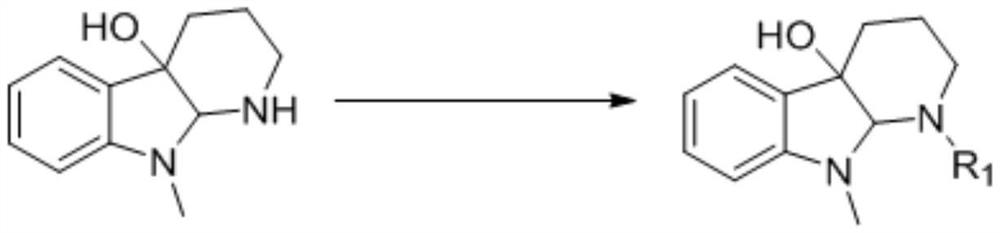
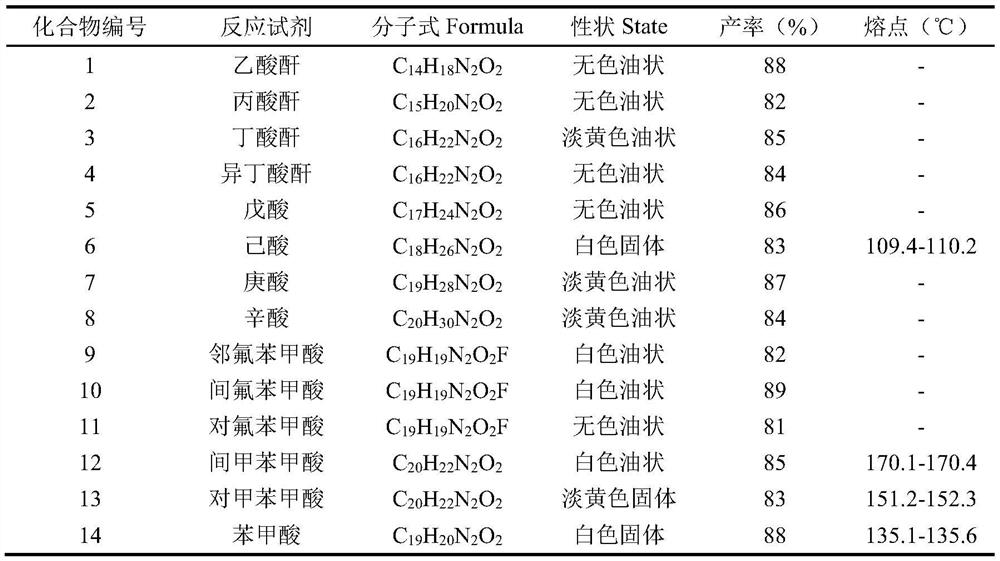
[0020] Weigh 200mg of the substrate 9-methyl-1,2,3,4,9,9a-6hydro-4aH-pyrido[2,3-b]indol-4a-ol and place in a 50mL dry round bottom In the flask, add 5mL of anhydrous pyridine and stir to dissolve, place the system in an ice-water bath at 0°C, and slowly add 0.07mL (0.72mmol) of acetic anhydride, the reaction reagent, to it dropwise. Detection, until the reaction is complete, add an appropriate amount of methanol dropwise to the reaction solution to quench the reaction, concentrate under reduced pressure to remove methanol and a small amount of pyridine, then extract 3 times with ethyl acetate, combine the organic phases, and successively wash with saturated CuSO 4 solution washed 3 times, saturated NaCl solution washed 3 times, anhydrous NaSO 4 dry. Concentrated under reduced pressure, the crude product was separated by column chromatography (PE:EA=6:1) to obtain 216 mg N-position acetylated derivative 1 (96%).
[0021] Character identificatio...
Embodiment 2
[0026] Synthesis
[0027] colorless oil, 1 H-NMR (400MHz, CDCl 3 ),δ7.34–7.04(m,2H),6.86–6.46(m,2H),5.30(s,1H),4.45–3.63(m,1H),3.29–3.17(m,1H),2.81(ddd ,J=13.1,8.3,4.8Hz,1H),2.64(d,J=25.6Hz,3H),2.58–2.34(m,2H),1.96–1.72(m,2H),1.67–1.24(m,2H ),1.17(dt,J=10.0,7.4Hz,3H). 13 C NMR (100MHz, CDCl 3 )δ 175.45(C), 149.54(C), 133.41(C), 129.52(CH), 129.33(CH), 122.04(CH), 119.06(CH), 108.34(CH), 85.36(C), 40.95(CH 2 ),37.20(CH 3 ), 33.29 (CH 2 ), 27.06(CH 2 ), 19.06, 9.60 (CH 3 ).
[0028] MS(ESI(+))calcd for C 15 h 20 N 2 o 2 [M+H] + :260.3; found: 261.0.
Embodiment 3
[0030]
[0031] Pale yellow oily, 1 H-NMR (400MHz, CDCl 3 ),δ7.26(s,2H),6.84–6.45(m,2H), 5.30(s,1H),4.44–3.62(m,1H),3.22(ddt,J=13.2,9.2,4.2Hz,1H ),3.01(s,1H), 2.80(ddd,J=13.1,8.3,4.8Hz,1H),2.63(d,J=32.5Hz,3H),2.55–2.23(m,2H), 1.96–1.74( m,2H),1.73–1.35(m,3H),0.96(dt,J=10.8,7.4Hz,3H). 13 C NMR (100MHz, CDCl 3)δ174.68(C), 149.54(C), 133.48(C), 129.27(CH×2), 122.03(CH), 119.02(CH), 108.30(CH), 85.35(C), 41.10(CH 2 ), 37.11 (CH 3 ), 35.31 (CH 2 ), 32.91 (CH 2 ), 19.40 (CH 2 ), 18.54 (CH 2 ),13.96.(CH 3 ).
[0032] MS(ESI(+))calcd for C 16 h 22 N 2 o 2 [M+H] + :274.4; found: 275.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com