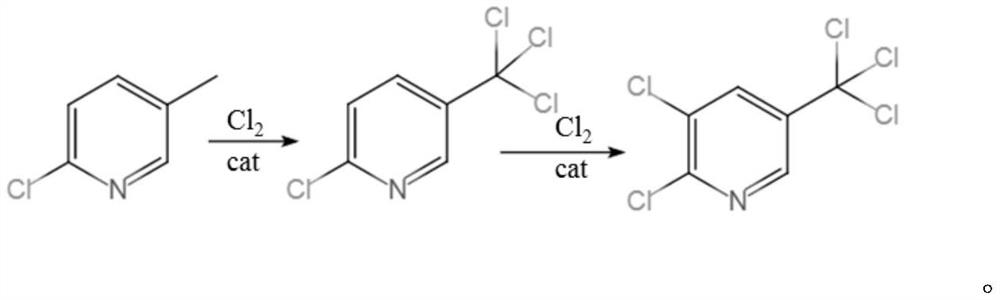
Synthesis method of 2, 3-dichloro-5-trichloromethylpyridine
A technology of trichloromethylpyridine and synthesis method, which is applied in the field of synthesis of 2,3-dichloro-5-trichloromethylpyridine, and can solve the problems of difficult post-processing, low utilization rate of chlorine gas, long chlorination time, etc. , to achieve a significant effect of chlorination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A kind of synthetic method of 2,3-dichloro-5-trichloromethylpyridine, comprises the following steps:
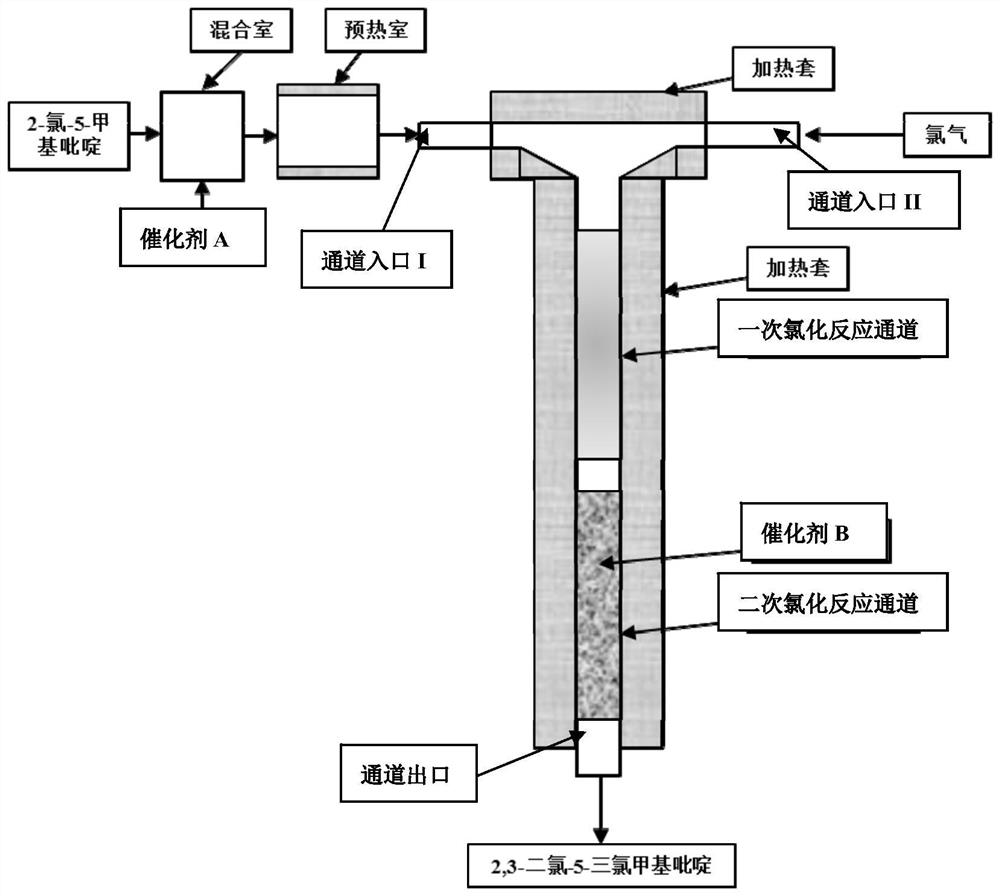
[0039] (1) Weigh 1.0g of ferric chloride in a flask, measure 100mL of deionized water, weigh 10g of activated carbon carrier and slowly add it to the flask, stir for 30min, ultrasonically disperse for 20min, soak at 60°C for 12h, and then put it in an oven at 120°C Dry for 12 hours, pass through a 200-mesh sieve after grinding, and put into a resistance furnace N 2 Roasting at 160°C for 4 hours under protection to obtain catalyst B supported by activated carbon;
[0040] Fill 1.0g of Catalyst B into the second half of the channel of the microreactor, and compact it with a microporous partition; after connecting the primary chlorination and secondary chlorination micro-reaction channels in series, connect the mixing chamber to the preheating chamber, and The heat chamber is connected to the channel inlet I of the microreactor, the channel inlet II of the microreactor i...
Embodiment 2
[0045] A kind of synthetic method of 2,3-dichloro-5-trichloromethylpyridine, comprises the following steps:
[0046] (1) Weigh 1.0g of ruthenium trichloride in a flask, measure 100mL of ionized water, weigh 10g of activated carbon carrier and slowly add it to the flask, stir for 30min, ultrasonically disperse for 20min, soak at 60°C for 12h, and then dry in an oven at 120°C 12h, pass through a 200-mesh sieve after grinding, and put it into a resistance furnace N 2 Roasting at 160°C for 4 hours under protection to obtain catalyst B supported by activated carbon;
[0047] Fill 1.0g of Catalyst B into the second half of the channel of the microreactor, and compact it with a microporous partition; connect the primary chlorination and secondary chlorination micro-reaction channels in series, and then connect the mixing chamber to the preheating chamber, The preheating chamber is connected to the channel inlet I of the microreactor, the channel inlet II of the microreactor is conne...
Embodiment 3
[0052] A kind of synthetic method of 2,3-dichloro-5-trichloromethylpyridine, comprises the following steps:
[0053] (1) Weigh 1.0g of tungsten hexachloride in a flask, measure 100mL of ionized water, weigh 10g of activated carbon carrier and slowly add it to the flask, stir for 30min, ultrasonically disperse for 20min, soak at 60°C for 12h, and then dry in an oven at 120°C 12h, pass through a 200-mesh sieve after grinding, and put it into a resistance furnace N 2 Roasting at 160°C for 4 hours under protection to obtain catalyst B supported by activated carbon;
[0054] Fill the second half of the microreactor channel with 1.0g of catalyst B, and compact it with a microporous partition; connect the primary chlorination and secondary chlorination microreaction channels in series, then connect the mixing chamber to the preheating chamber, and preheat The heat chamber is connected to the channel inlet I of the microreactor, the channel inlet II of the microreactor is connected t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com