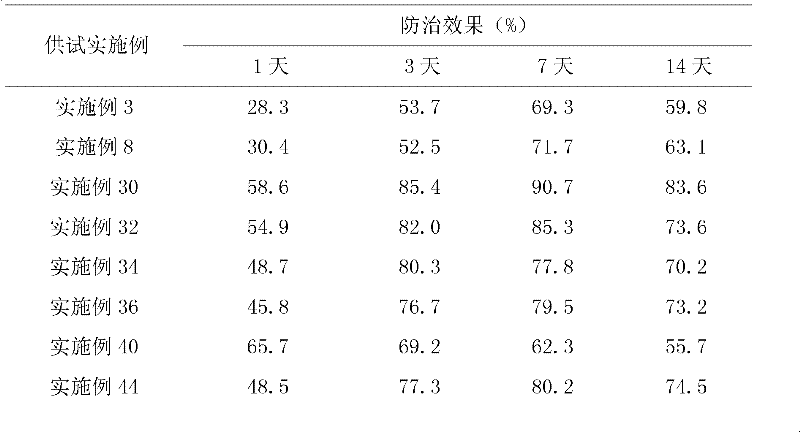
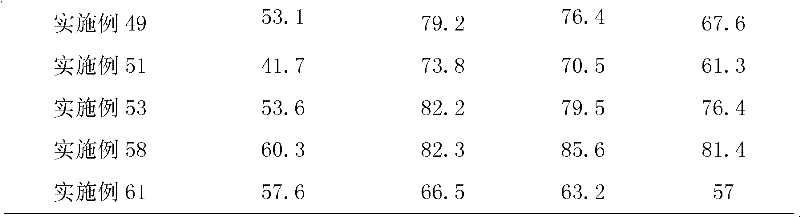
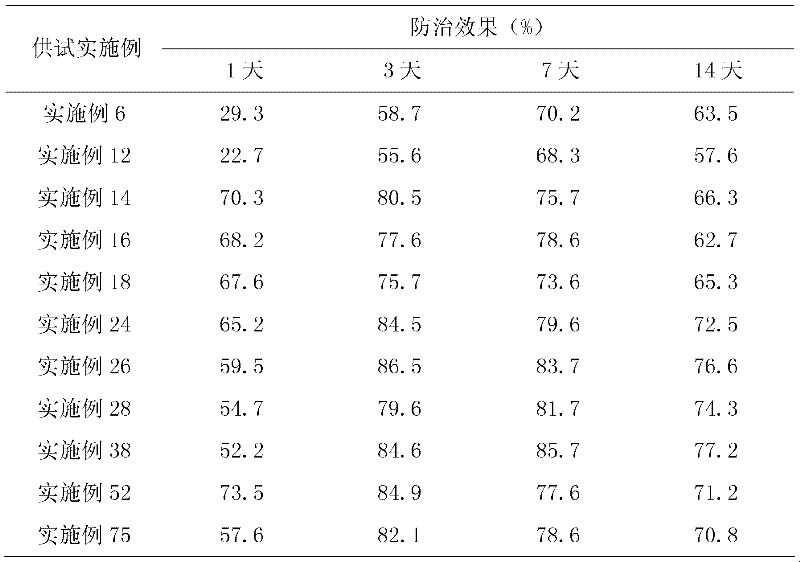
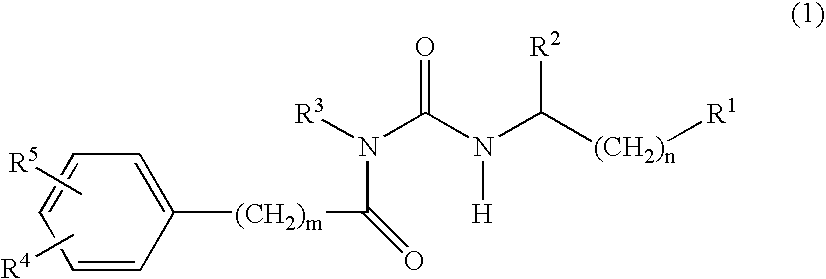
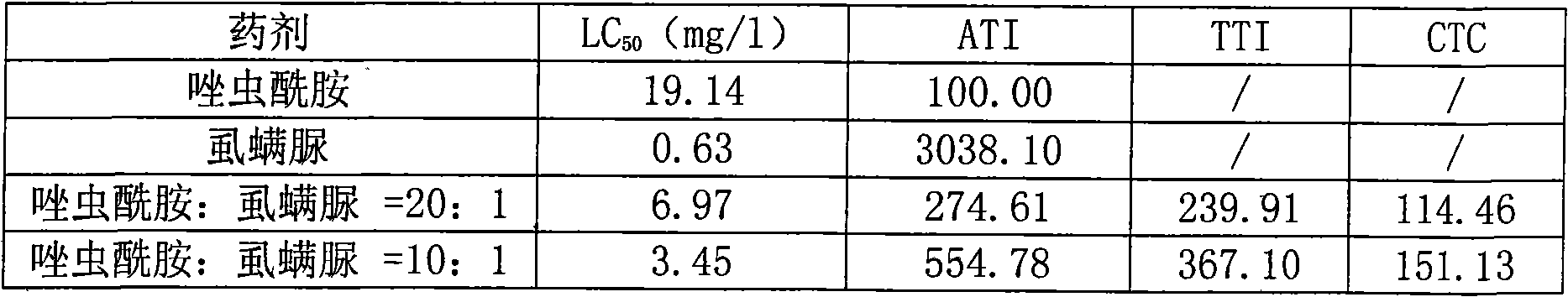
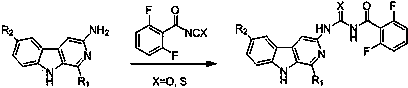
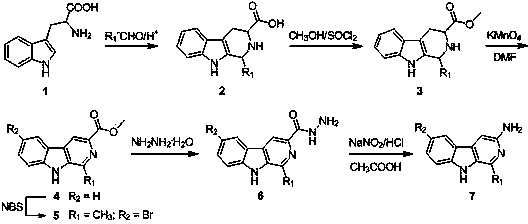
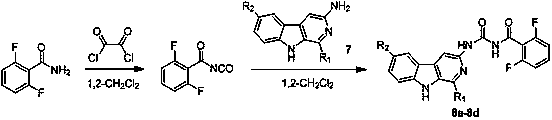
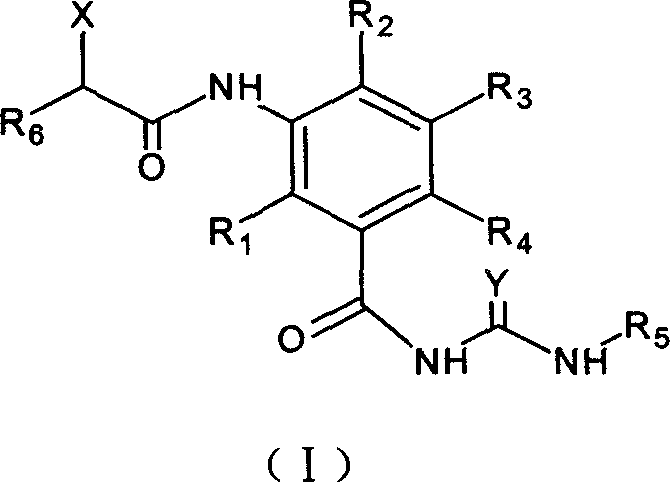
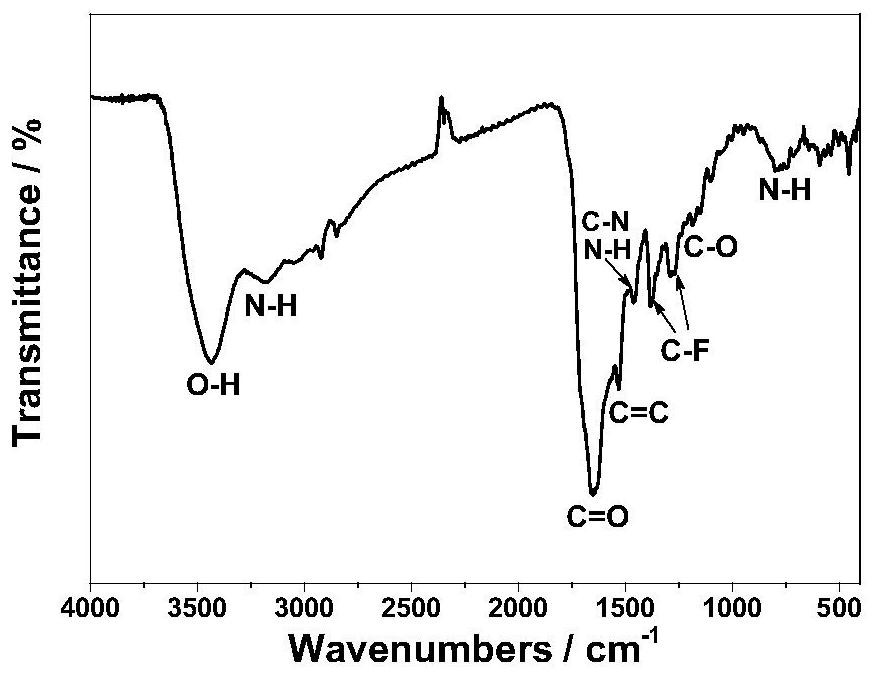
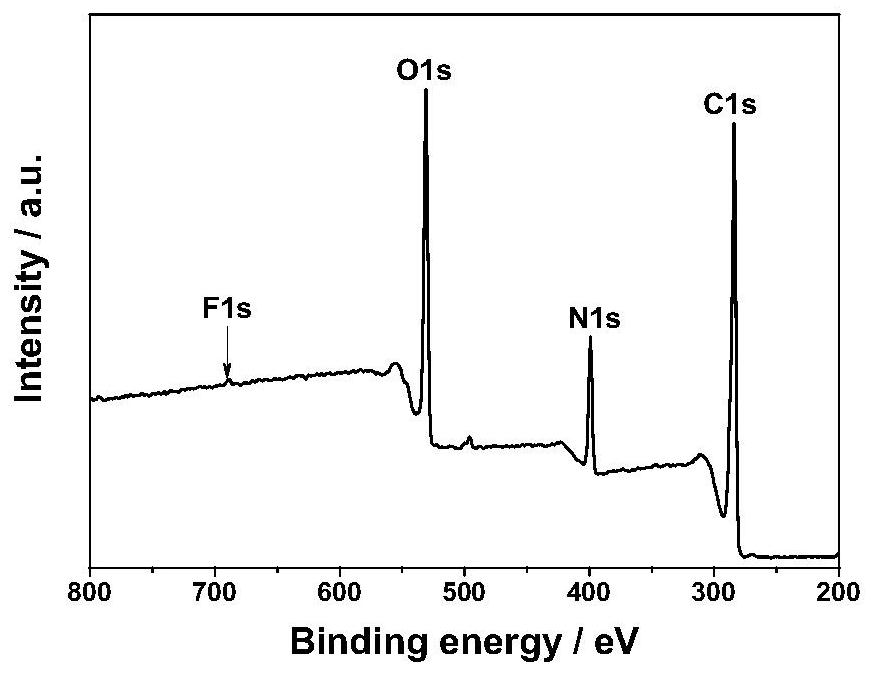
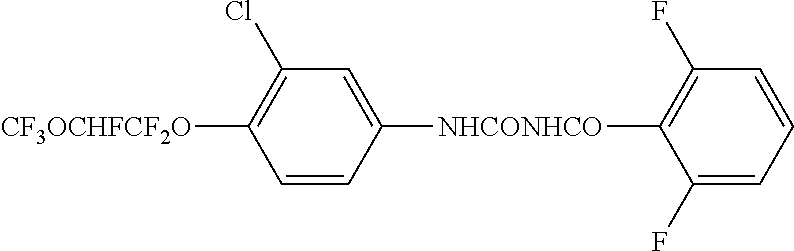
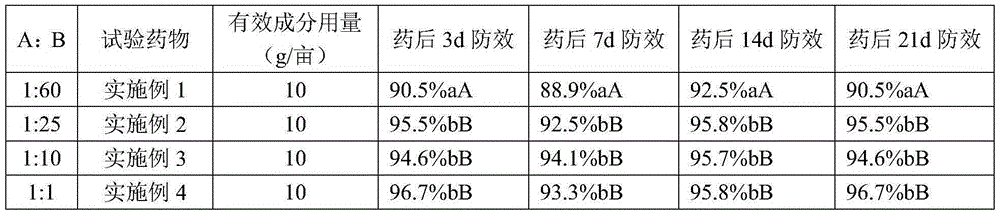
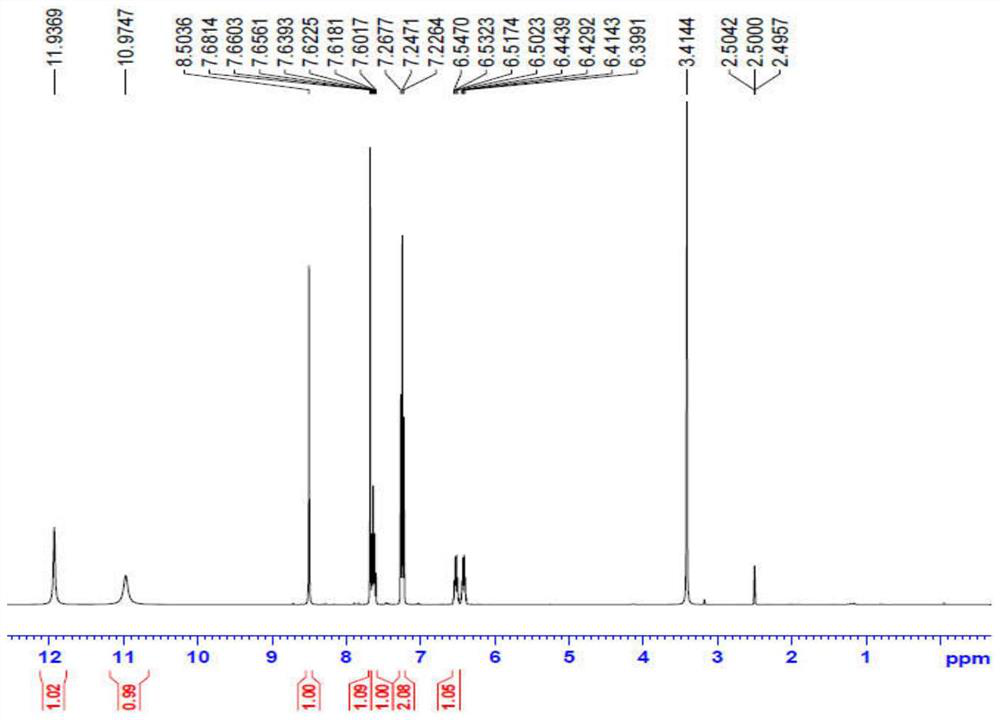
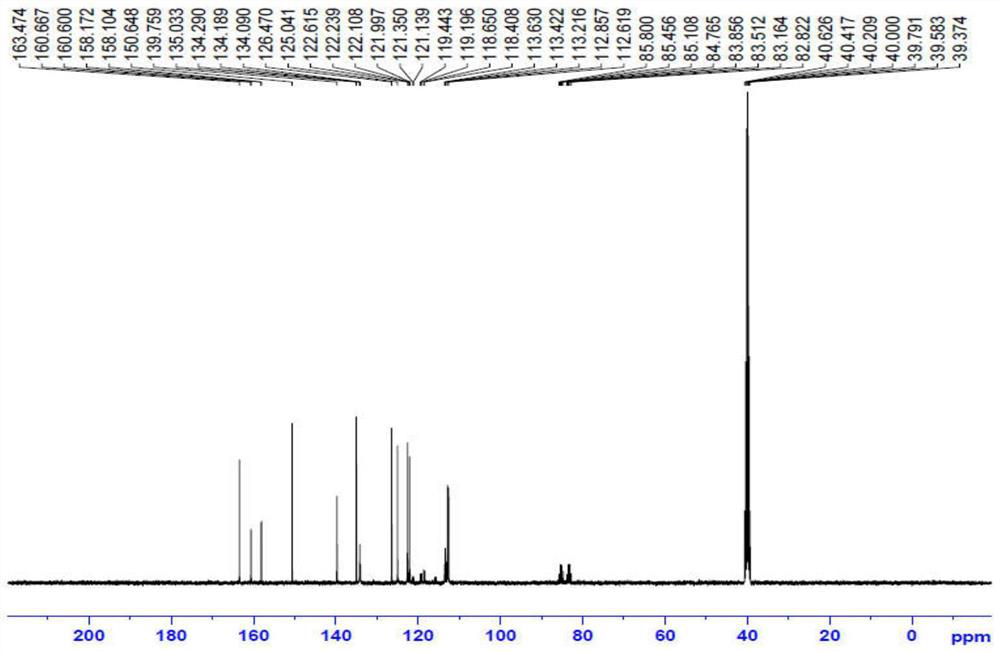
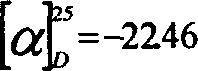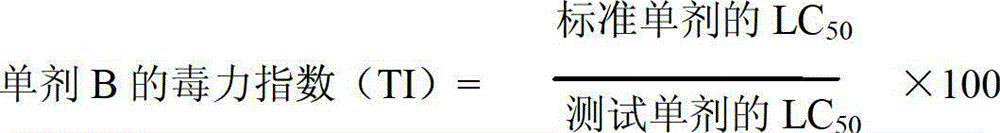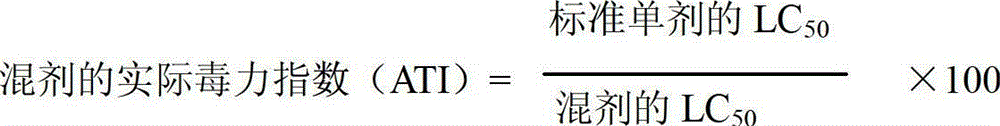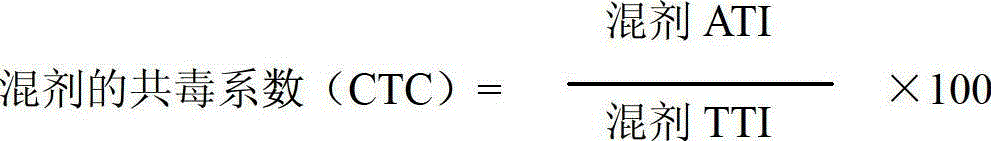Patents
Literature
59 results about "Benzoylurea" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
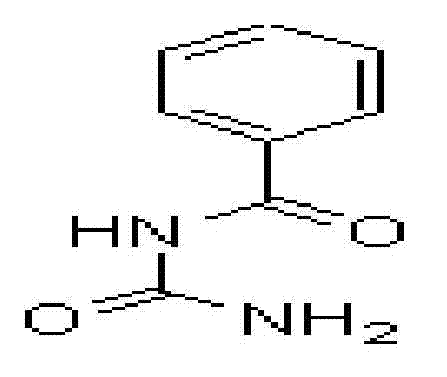
Benzoylureas are chemical derivatives of N-benzoyl-N′-phenylurea (benzoylurea). They are best known for their use as insecticides. They act as insect growth regulators by inhibiting synthesis of chitin in the insect's body.
Ultralow volume liquid containing benzoylurea insecticide
InactiveCN102379282AEasy to processHigh efficiency of pesticide applicationBiocideAnimal repellantsNicotiana tabacumAdjuvant
The invention relates to the field of pesticides, in particular to ultralow volume liquid containing benzoylurea insecticide, which comprises active ingredients, solvent and adjuvant. The active ingredient I is optional one of lufenuron, diflubenzuron, triflumuron, chlorfluazuron and chlorbenzuron belonging to benzoylurea, and the active ingredient II is insecticide of another type of function mechanism. The ultralow volume liquid containing benzoylurea insecticide has the advantages of simplicity in processing, high efficiency, water saving, long-term effect, a target being not easy to resist the insecticide, low environmental pollution, synergy and the like. Application of ultralow volume spray, low volume spray or ultralow volume static spray is well effective in controlling ostrinia furnacalis, sugarcane borer, locust, peach fruit borer, lesser green leaf hopper, striped flea beetle, bean fly, diamond back moth, prodenia litura, cabbage caterpillar, leaf beetle, thrip, leaf miner, inchworm, red spider and the like, and the ultralow volume liquid containing benzoylurea insecticide can be used for effectively controlling various pests on wheat, maize, vegetables, tobacco plants, tea plants, fruit trees, cotton plants, woods and sugarcanes.
Owner:GAUNGXI TIANYUAN BIOCHEM
Alpha-helical mimetics
Benzoyl urea derivatives that are alpha helical peptides mimetics that mimic BH3-only proteins, compositions containing them, their conjugation to cell-targeting-moieties, and their use in the regulation of cell death are disclosed. The benzoyl urea derivatives are capable of binding to and neutralizing pro-survival Bcl-2 proteins. Use of benzoyl urea derivatives in the treatment and / or prophylaxis of diseases or conditions associated with deregulation of cell death are also described.
Owner:WALTER & ELIZA HALL INST OF MEDICAL RES
Synergistic insecticidal composition and application thereof
InactiveCN101653123AExtended service lifeDelays resistance developmentBiocideAnimal repellantsBenzoylphenylureaToxicology
The invention relates to a pesticide composition. The composition is characterized by comprising tolfenpyrad (A) and another benzoylphenylurea pesticide active constituent (B), wherein the (B) can belufenuron, hexaflumuron, ehlorfluazuron, flufenoxuron or chlorbenzuron. The invention also relates to an application of the pesticide composition in preventing and curing pests of crops.
Owner:SHENZHEN NOPOSION AGROCHEM
Benzoylurea insecticide and Thiamethoxam compound preparation
InactiveCN101204156AWith preventionEffective controlBiocideAnimal repellantsOrder LepidopteraThiamethoxam
The invention relates to a pesticide which is a composite preparation formulated by benzoylphenylurea insecticide and thiamethoxam used for controlling lepidoptera, coleopteran, thysanoptera and homopterous pests. Effective components of the preparation comprise 0.1-30.0 percent of the benzoylphenylurea insecticide and 0.1-75.0 percent of the thiamethoxam; the benzoylphenylurea insecticide relates to any one of triflumuron, mieyouniao, hexaflumuron and diflubenzuron; the allowance comprises promoter, filler, solvent or water which is often used in the pesticide. The pesticide has effects of stomach toxicity, contact killing and absorption and is characterized by fast speed and long persistent period. The pesticide can effectively control the lepidoptera, coleopteran and thysanoptera pests and has a high effect on homopterous pests. The pesticide has a good controlling effect on piercing-sucking pests such as aphid, planthopper, leafhopper and whitefly. The complex formulation of the benzoylphenylurea insecticide and the thiamethoxam can kill pests more thoroughly than single preparation of common benzoylphenylurea insecticide and thiamethoxam and has a longer effect.
Owner:通化绿地农药化学有限公司
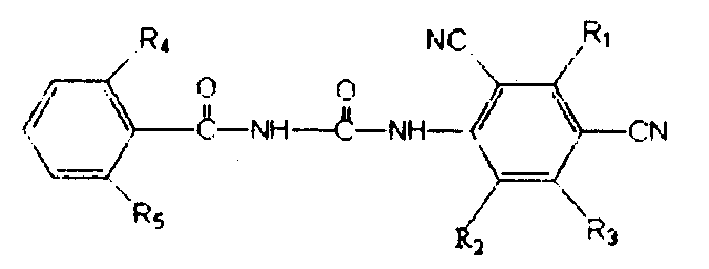
Benzoylurea Compounds and Use Thereof
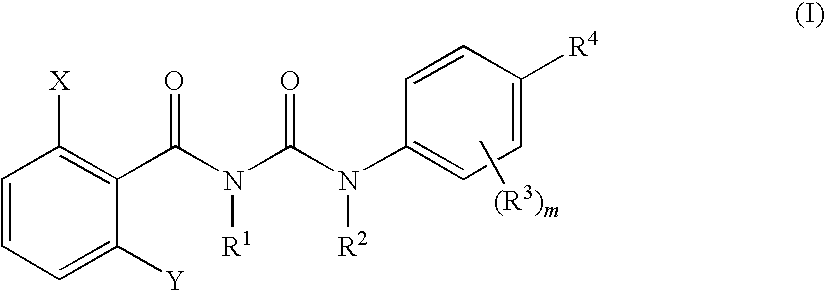
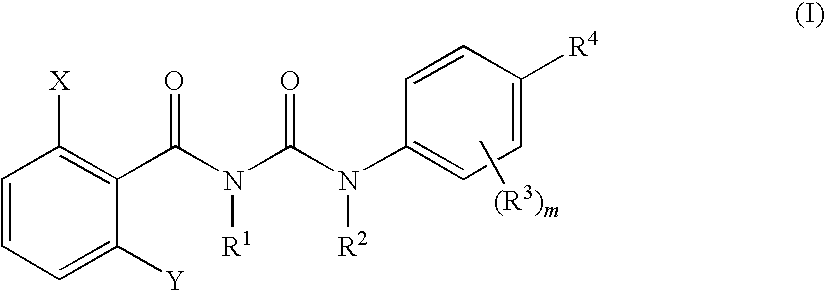
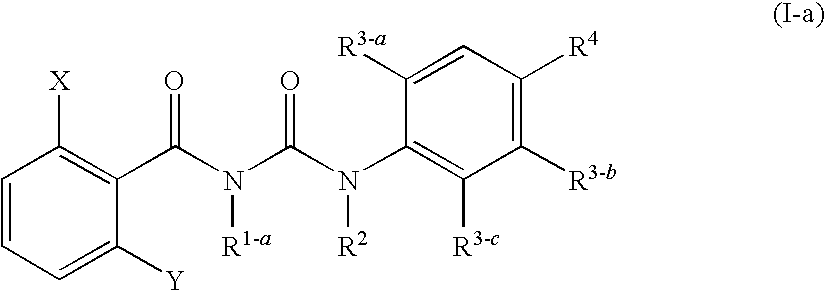
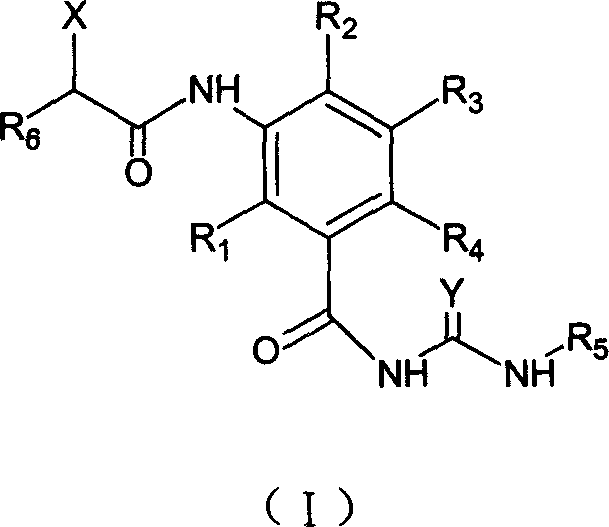
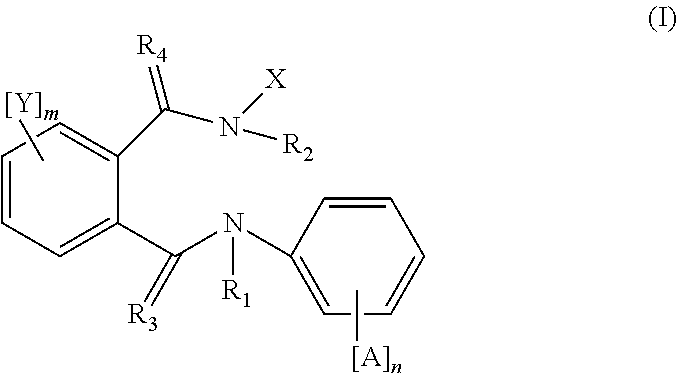
The present invention relates to a benzoylurea compound represented by formula (I):wherein, X and Y represent a fluorine atom or a chlorine atom, R1 represents a lower alkyl group or the like, R2 represents a lower alkyl group, R3 represents a halogen atom or the like, R4 represents an alkylthio group optionally substituted with one or more of halogen atoms, or a salt thereof, and use thereof for controlling pests and the like.
Owner:SUMITOMO CHEM CO LTD
Banisterine benzoyl urea compounds and preparation method and application thereof
ActiveCN104628723AHigh insecticidal activityStrong inhibitory activityOrganic chemistryFungicidesFragariaCitrus volkameriana
The invention discloses banisterine benzoyl urea compounds and a preparation method and application thereof. The banisterine benzoyl urea compounds have a structural formula I shown in the specification; in the formula I, R1 can be methyl, phenyl, 3,4,5- triethoxy phenyl, p-methoxyphenyl or p-chlorphenyl, R2 can be H or Br, and X can be O or S. The banisterine benzoyl urea compounds have an outstanding insecticidal activity for Culex fatigans, prodenia litura and Chilo suppressalis; a part of the compounds are identical to a control insecticide Dibenzoyl-1-tert-butylhydrazine in term of activity; the banisterine benzoyl urea compounds have good inhibitory activity for rice sheath blight disease, alternaria solani, Collectotrichum musae, grey mould fruit rot of strawberry and sour rot pathogenic bacteria of citrus; a part of the compounds have better inhibitory activity for the above five phytopathogens than validamycin. The banisterine derivatives are simple in structure and easy to synthesize; the synthesis process is simple, the product purity is high; the banisterine benzoyl urea compounds and the preparation method thereof are suitable for large-scale industrial popularization and application.
Owner:SOUTH CHINA AGRI UNIV
3-amido substituted nenzoyl urea compound, and its antitumor action
InactiveCN1850794AEnhance killing activityOrganic chemistryPharmaceutical delivery mechanismToxicitySide chain
This invention relates to new style 3-acylamino substituted benzoyl urea kind compound. On base of deep research of structure activity, benzene ring mother nuclide, 1 bit acyl urea side chain and 3 bit acyl urea side chain are differently replaced to synthesize series derivant. Good compound is selected from the derivant, and it has strong activity revealed by anti tumor experiment, and toxicity is low, so it is convenient to synthesize, and anti medicine capability is hard form, so it has good application prospect.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Cycloxaprid insecticidal composition
ActiveCN103843802AHigh activityGood control effectBiocideAnimal repellantsSemicarbazoneCarbamate insecticide
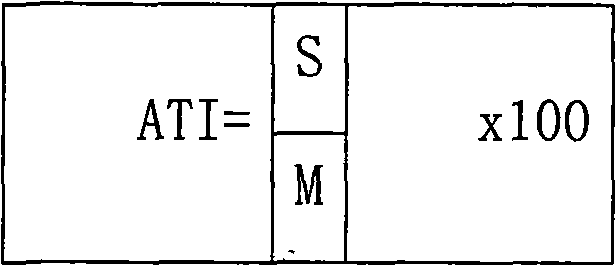
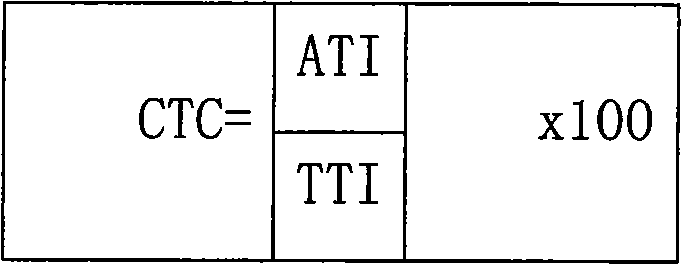
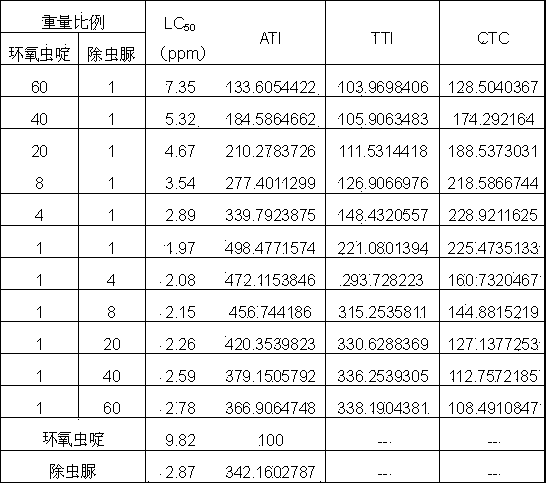
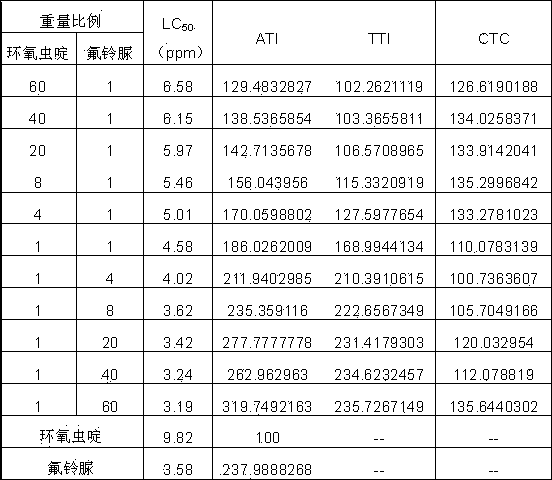
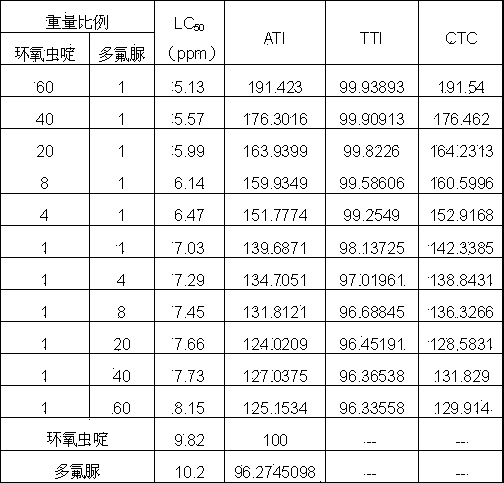
A provided cycloxaprid composition insecticide comprises cycloxaprid and a second active ingredient with a weight ratio of 60:1-1:60. The second active ingredient is any one or a mixture of any several selected from benzoylurea growth regulators, pyridazinone growth regulators, semicarbazone insecticides, pyridinecarboxamide insect growth regulators and carbamate insecticides. The cycloxaprid composition insecticide has the co-toxicity coefficient (CTC) obviously higher than 100, has obvious synergic effect and additive effect, and helps to obviously reduce the use amount of pesticides.
Owner:SHANGHAI SHENGNONG PESTICIDE
Pesticide composition of chlorantraniliprole and benzoyl urea insecticides
InactiveCN104705329AExtended service lifeDelays resistance developmentBiocideAnimal repellantsTreatment effectOrder Lepidoptera
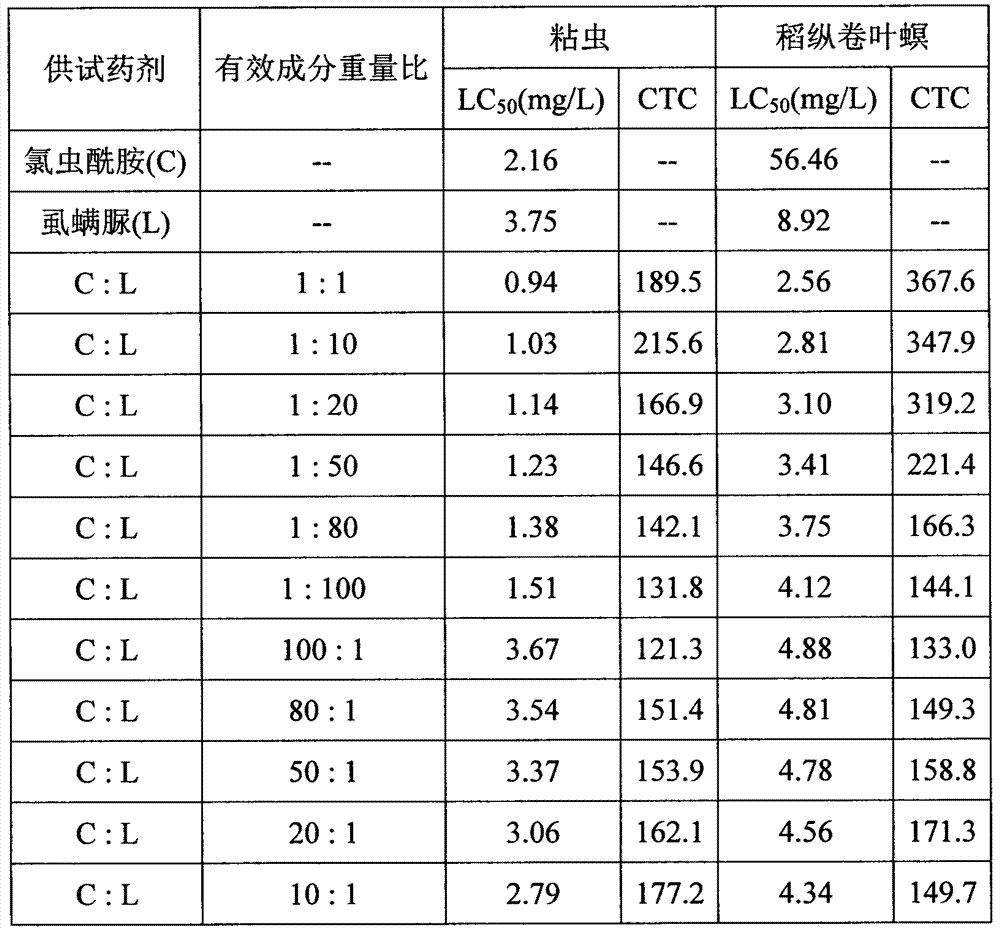
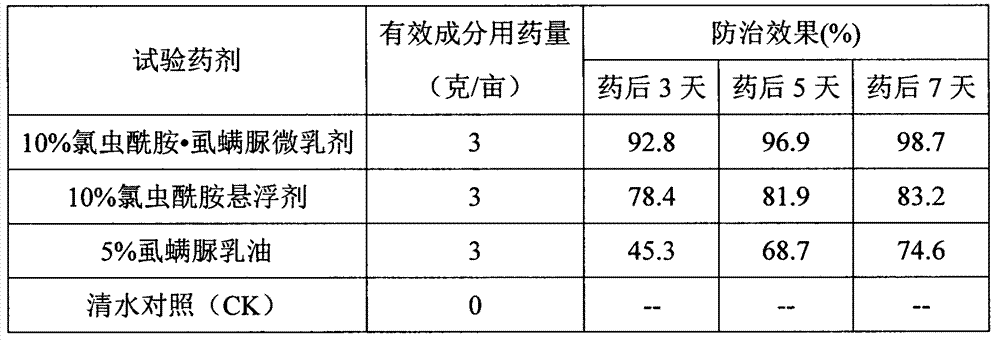
The invention relates to a mixed pesticide preparation containing a benzoyl urea insecticide. The mixed pesticide preparation comprises the effective components, namely chlorantraniliprole and benzoyl urea, wherein the benzoyl urea insecticide can be lufenuron, hexaflumuron, flufenoxuron, chlorfluazuron, chlorbenzuron, diflubenzuron or teflubenzuron and the like; the weight percent of the benzoyl urea is 1%-20%; the weight percent of the benzoyl urea insecticide is 1%-30%; and the preparation can be prepared into missible oil, a microemulsion, an emulsion in water, water dispersible granules, wettable powder or a suspending agent. According to the insecticidal composition, relatively high synergetic effects can be generated; resistance development of a synthesis inhibitor of benzoyl urea chitin is retarded; the service life of medicines is prolonged; prevention and treatment effects on lepidoptera pests are improved; and compared with that of the benzoyl urea insecticide, the fast-acting property is obviously improved.
Owner:TIANJIN AGRICULTURE COLLEGE
Benzoyl Urea Derivatives
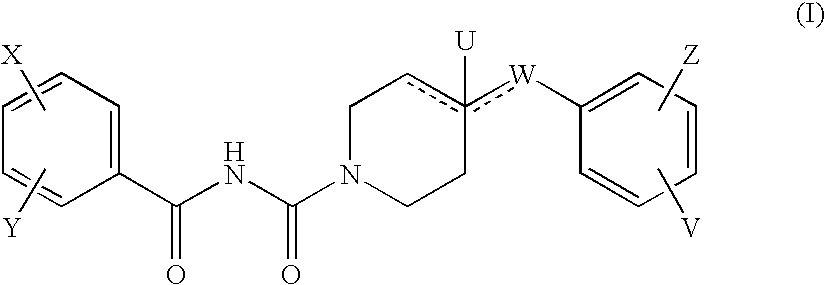
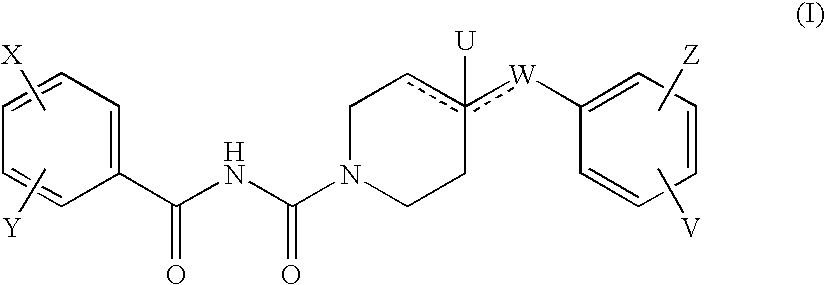
The new benzoyl urea derivatives of formula (I) wherein the meaning of X and Y independently are hydrogen atom, hydroxy, benzyloxy, amino, nitro, C1-C4 alkylsulfonamido optionally substituted with a halogen atom or halogen atoms, C1-C4 alkanoylamido optionally substituted with a halogen atom or halogen atoms, C1-C4 alkoxy, aroyl-carbamoyl optionally substituted with halogen atom or C1-C4 alkyl or C1-C4 alkoxycarbonyl group, or the neighboring X and Y groups optionally form together with one or more identical or different additional hetero atom and —CH═ and / or —CH2— groups an optionally substituted 4-7 membered homo- or heterocyclic ring, preferably morpholine, pyrrole, pyrrolidine, oxo- or thioxo-pyrrolidine, pyrazole, pyrazolidine, imidazole, imidazolidine, oxo- or thioxo-imidazole or imidazolidine, 1,4-oxazine, oxazole, oxazolidine, triazole, oxo- or thioxo-oxazolidine, or 3-oxo-1,4-oxazine ring, V and Z independently are hydrogen or halogen atom, cyano, C1-C4 alkyl, C1-C4 alkoxy, trifluoromethyl, hydroxy or optionally esterized carboxyl group, W is oxygen atom, as well as C1-C4 alkylene, C2-C4 alkenylene, aminocarbonyl, —NH—, —N(alkyl)-, —CH2O—, —CH2S—, —CH(OH)—, —OCH2— group, wherein the meaning of alkyl is a C1-C4 alkyl group—, when the dotted bonds () represent simple C—C bonds then U is hydroxy group or hydrogen atom or when W is C1-C4 alkylene or C2-C4 alkenylene group, then one of the dotted bonds () can represent a further double C—C bond and in this case U means an electron pair, which participate in the double bond and optical antipodes, racemates and the salts thereof are highly effective and selective antagonists of NMDA receptor, and moreover most of the compounds are selective antagonist of NR2B subtype of NMDA receptor. Furthermore objects of the present invention are the pharmaceutical compositions containing new benzoyl urea derivatives of formula (I) or optical antipodes or racemates or the salts thereof as active ingredients and processes for producing these compounds and pharmaceutical compositions.
Owner:RICHTER GEDEON VEGYESZETI GYAR RT
Nitrogen-doped fluorescent carbon dot based on citric acid and benzoylurea as well as preparation method and application thereof
ActiveCN112251223AGood biocompatibilityHigh reactivityNanoopticsNano-carbonQuantum yieldFreeze-drying
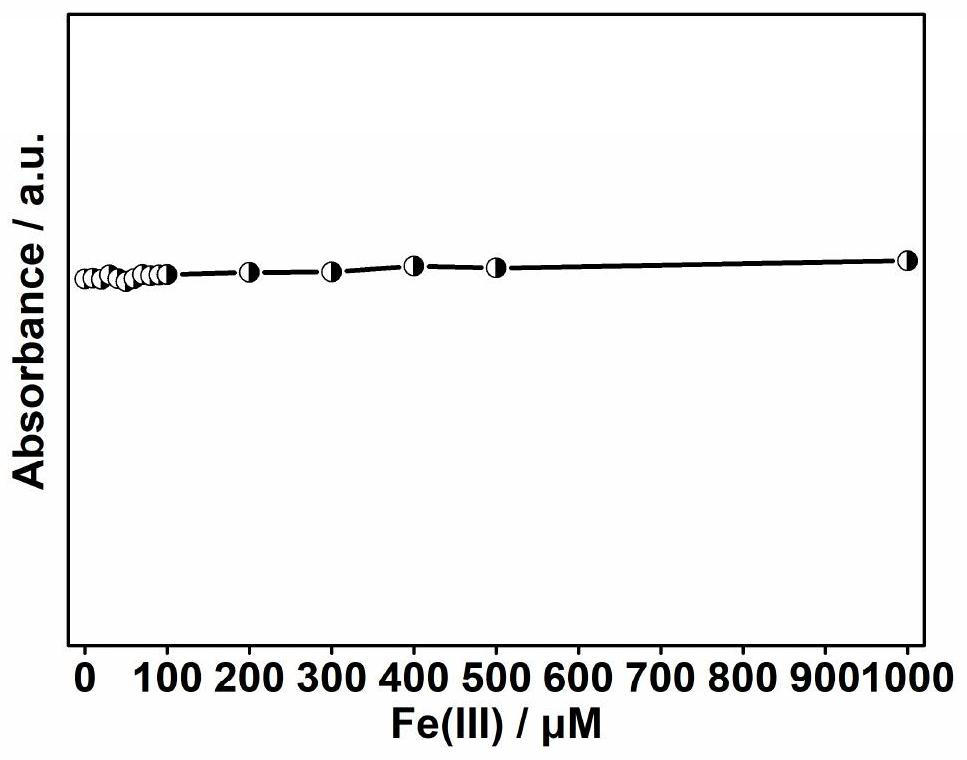
The invention discloses a nitrogen-doped fluorescent carbon dot based on citric acid and benzoylurea, which is prepared from citric acid and benzoylurea as raw materials, and a preparation method comprises the following steps: (1) mixing citric acid and benzoylurea, and carrying out high-temperature solid-phase preparation; (2) sequentially cooling, dissolving and filtering reactants obtained in the step (1); and (3) dialyzing the filtrate obtained in the step (2), and freeze-drying to obtain the nitrogen-doped fluorescent carbon dots. The application is as follows: the carbon dot is used forpreparing a fluorescence sensor for detecting Fe (III). The preparation and post-treatment method has the advantages that the operation is simple, the surface of the obtained carbon dot is rich in amino and hydroxyl, the carbon dot is soluble in water, the fluorescence quantum yield is high, and the carbon dot has a selective sensing effect on Fe (III) in an aqueous solution and has a wide application prospect.
Owner:XIHUA UNIV +1
Insecticidal composition containing oxymatrine and benzoylurea components
ActiveCN102939978ADelays resistance developmentExtended service lifeBiocideAnimal repellantsCotton bollwormSpodoptera
The invention relates to an insecticidal composition containing oxymatrine and benzoylurea components. The mass ratio of the oxymatrine to benzoylurea insecticides is (1-40): (40-1). The benzoylurea insecticides are one or more of hexaflumuron, flufenoxuron, chlorfluazuron, lufenuron, novaluron, chlorbenzuron, diflubenzuron and teflubenzuron. The insecticidal composition can be used for preventing beet armyworms, rice leaf folders, cotton bollworms, plutella xylostellas, cabbage caterpillars, prodenia litura, apple skin worms, whiteflies and red spider mites.
Owner:HAINAN ZHENGYE ZHONGNONG HIGH TECH
Benzoyl urea insecticide and buprofezin compound preparation
InactiveCN101455204AGood prevention effectIncrease virulenceBiocideAnimal repellantsInsect pestBULK ACTIVE INGREDIENT
The invention relates to an agricultural chemical that is a compositional preparation of benzoylureas insecticide and buprofezin having a better ability of preventing insect pests such as aphids, plant hopper, leafhopper, whitefly and the like.The active ingredients of the compositional preparation is prepared by the following raw medicines of percentage by weight:benzoylureas insecticide of 0.1%-30.0%, buprofezin of 0.1%-90.0% and the rest are auxiliary agent, filling material, solvent or water that are commonly used in agricultural chemicals. Comparing with common benzoylureas insecticide and single dose of buprofezin, the insect disinfestation effect of active ingredients of the compositional preparation is more thorough and the action is more lasting.A test indicates that the compositional preparation has an upper virulence to nymphae of brown back rice plant hopper and shows obvious synergism.
Owner:通化绿地农药化学有限公司
Fruit tree miridae pesticide and preparation method thereof
The invention discloses a fruit tree miridae pesticide which is characterized in that the pesticide is mainly composed of the components of: 2 parts of benzoylurea, 1-5 parts of bifenthrin, 1-5 parts of thiamethoxam, 30-40 parts of pyrethrum, 20-40 parts of snakegourd root, 30-50 parts of rehmannia root, 10-20 parts of rangooncreeper fruit, and 10-20 parts of chinaberry bark. The invention also discloses a preparation method of the fruit tree miridae pesticide. Compared to prior arts, the fruit tree miridae pesticide provided by the invention is harmless to human and livestock, and causes no phytotoxicity to crops. The pesticide has long-lasting period, and can effectively kill fruit tree miridae. With the fruit tree miridae pesticide, pesticide efficacy can be improved, the possibility of miridae pesticide resistance can be reduced, and environment pollution can be reduced.
Owner:卞佳林
Composition for controlling house insect pest and method for controlling house insect pest
InactiveUS20050215597A1High activityReduce doseBiocideDead plant preservationHydrazoneCompound (substance)
Owner:ISHIHARA SANGYO KAISHA LTD
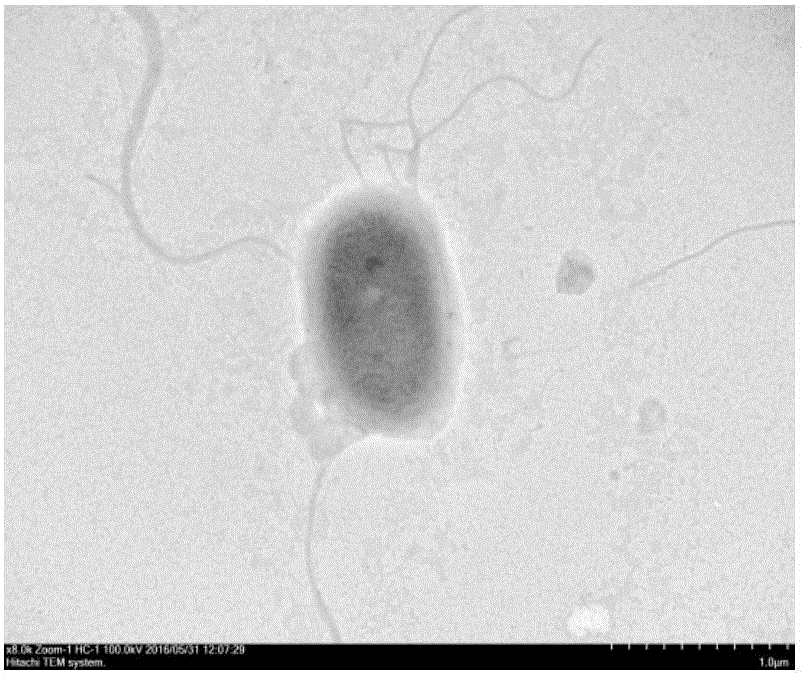
Benzoyl urea insecticide degrading bacteria, bacterial agent by using same and application of benzoyl urea insecticide degrading bacteria
ActiveCN106085899ASolve the hazardEliminate residueBacteriaMicroorganism based processesInsecticide degradationMicrobiology
The invention discloses a benzoyl urea insecticide degrading bacterial strain M6. The benzoyl urea insecticide degrading bacterial strain M6 is identified as achromobacter dolens, is preserved in the China Center for Type Culture Collection on May 11, 2016, and has a preservation number of CCTCC NO: M 2016257. The invention further discloses an application of the bacterial strain M6 in production of a benzoyl urea insecticide degrading bacterial agent, the degrading bacterial agent can degrade a variety of benzoyl urea insecticides such as hexaflumumron, diflubenzuron and chlorbenzuron, and the degradation rate of residual hexaflumumron, diflubenzuron and chlorbenzuron in soil or a water body environment can be up to 90% or above in a short time; the degrading bacterial agent can be produced by using general fermentation equipment of the fermentation industry, and has the advantages of low production cost, convenient use and good removal effects.
Owner:ENVIRONMENT & PLANT PROTECTION INST CHINESE ACADEMY OF TROPICAL AGRI SCI
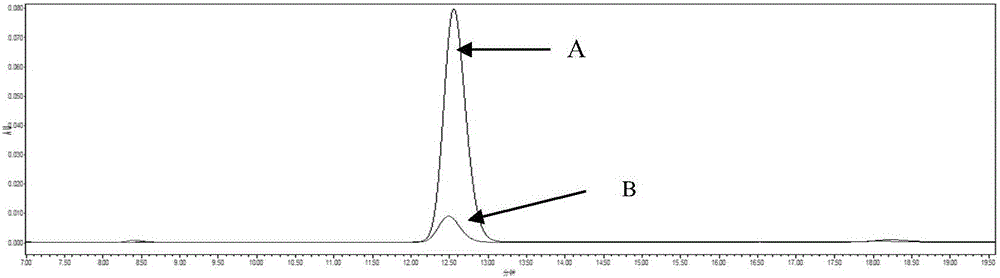
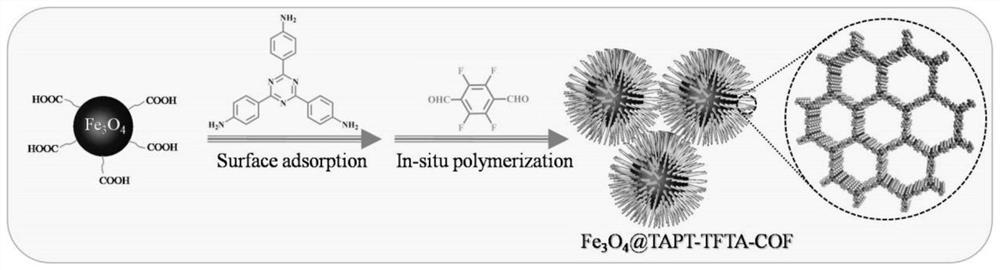
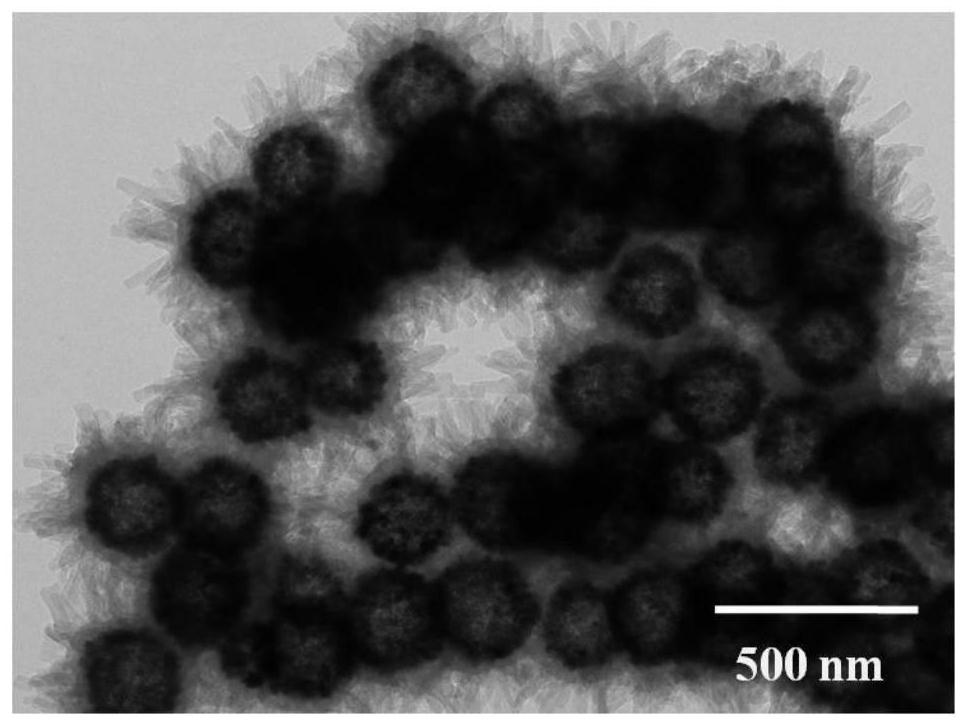
Magnetic fluorinated covalent organic framework material as well as preparation method and application thereof
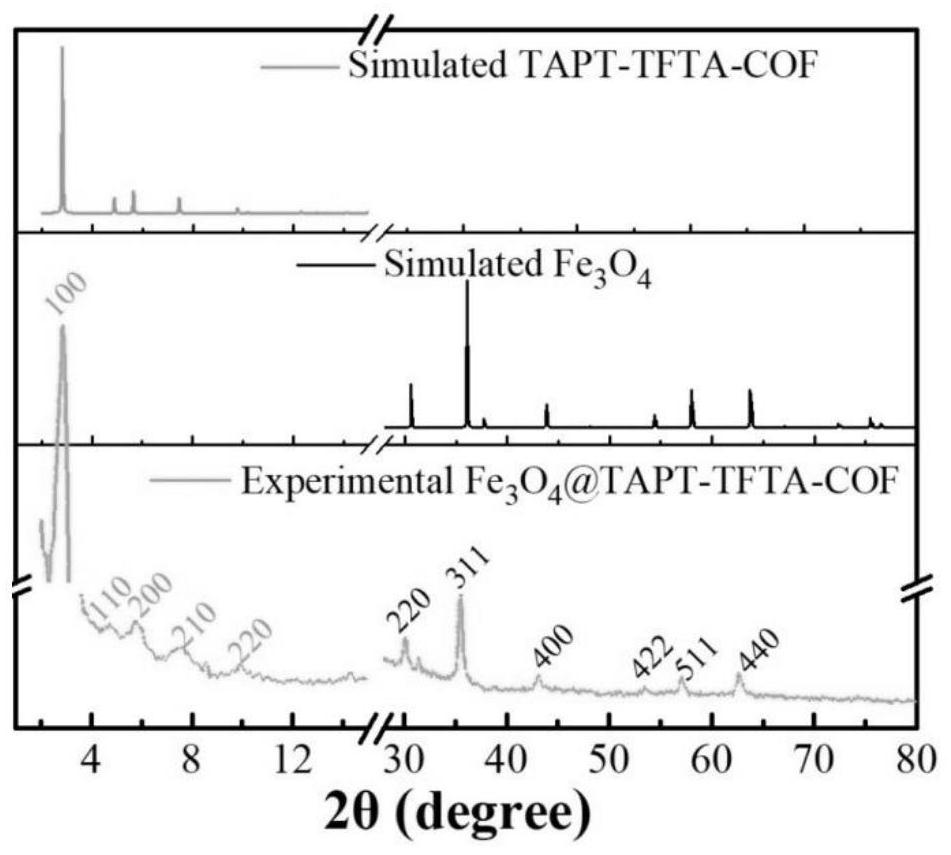
ActiveCN113717337AReduce dosageReduce extraction timeComponent separationCarboxyl radicalStructural formula
The invention provides a magnetic fluorinated covalent organic framework material and a preparation method and application thereof. The structural formula of the magnetic fluorinated covalent organic framework material is Fe3O4-coated TAPT-TFTA-COF, and the magnetic fluorinated covalent organic framework material is of a core-shell structure prepared by taking carboxylated Fe3O4 nanoparticles as a core and taking a covalent structure prepared from 2,4,6-tri(4-aminophenyl)-1,3,5-triazine and 2,3,5,6-tetrafluoroterephthalaldehyde as monomers through polycondensation, and the surface of the shell layer is in a sea urchin shape. The magnetic fluorinated covalent organic framework material disclosed by the invention is used for magnetic solid-phase extraction of benzoylurea pesticides, shows strong adsorption selectivity and high separation and enrichment efficiency, and has the remarkable advantages of being small in adsorbent dosage, short in extraction time, capable of being repeatedly used and capable of reducing the detection cost.
Owner:SHANDONG ANALYSIS & TEST CENT
Emamectin benzoate-diflubenzuron compound pesticide
InactiveCN102550578AHigh activityImprove contact abilityBiocideAnimal repellantsPreservativeEmamectin benzoate
The invention provides an emamectin benzoate-diflubenzuron compound pesticide which comprises the following components in percentage by weight: 0.3 to 1.5 percent of emamectin benzoate, 5 to 20 percent of diflubenzuron, 2 to 6 percent of suspension auxiliary agent, 1 to 12 percent of wetting dispersant reagent, 1 to 5 percent of antifreezing agent, 0.1 to 1 percent of thickening agent, 0.2 to 0.5 percent of defoaming agent, 0.1 to 2 percent of antiseptics, and the balance of water which is added until reaching 100 percent of weight. The pesticide is formed by compounding benzoylurea pesticide diflubenzuron and emamectin benzoate, belongs to a compound pesticide, and has the advantages of strong quick-action, low residue, high inset and egg killing activity.
Owner:安阳市安林生物化工有限责任公司
High-performance and friendly-environmental pesticide compound composition
InactiveCN102224826ASynergistic effect is obviousReduce pollutionBiocideAnimal repellantsWater dispersibleAdjuvant
The invention relates to a high-performance and friendly-environmental pesticide compound composition. The pesticide compound composition is characterized in that: effective components of the pesticide compound composition comprise a clothianidin amide compound and a benzoylurea compound, wherein a weight percent of the clothianidin amide compound to the benzoylurea compound is 1-80:80-1, preferably 1-40:40-1; a total mass percentage content of the effective components in pesticide compound composition is 1%-90%, preferably 10-50%, and the balance is agrochemically- workable and agrochemically-acceptable adjuvants; the pesticide compound composition can be prepared in an aqueous emulsion, a microemulsion, a cream, a suspension, a wettable powder or a water dispersible granule; the pesticide compound composition adopts the clothianidin amide and the neonicotine insecticide as the effective components for controlling homopteran pests and thysanoptera pests, such as rice leaf roller, diamond-back moth, prodenia litura, cotton bollworm or spodoptera exigua, thrips and the like.
Owner:HAILIR PESTICIDES & CHEM GRP
Efficient insecticide containing benzoy urea, azamethiphos and thiamethoxam
InactiveCN104273166ASynergistic effect is obviousReduce pollutionBiocideAnimal repellantsPesticide residueThiamethoxam
Owner:QINGDAO YONGTONG ELEVATOR ENG
Synergistic compound pesticide contg. trans-cypermethrin and benzoyl urea
InactiveCN1911027AReduce pollutionIncrease resistanceBiocideAnimal repellantsCypermethrinDrug resistance
A synergistic composite insecticide for preventing and treating rice borer contains trans-cypermethrin, benzoylurea, assistant and excipient.
Owner:NANJING NO 1 PESTICIDE GRP
Red light carbonized polymer dot based on citric acid and benzoylurea and preparation method and application thereof
ActiveCN113234438AEasy to prepareExcellent fluorescence propertiesIn-vivo testing preparationsEnergy efficient lightingCarbonizationIn vivo
The invention discloses a red light carbonized polymer dot based on citric acid and benzoylurea and a preparation method and application thereof, the raw materials of the red light carbonized polymer dot based on citric acid and benzoylurea comprise citric acid and benzoylurea, and the mass ratio of citric acid to benzoylurea is (0.5-3): (1-6). The N, N-dimethylformamide is used as a solvent, ammonium fluoride is used as a fluorine source additive, the surface polymer state is enriched while the carbon nucleus crystalline state of the RCBCPDs is enhanced, and the RCBCPDs are promoted to have a larger multi-photon absorption cross section after fluorine is introduced, so that a stronger fluorescence imaging effect is displayed in vitro and in vivo. On the basis of a citric acid and benzoylurea carbon dot preparation system, the carbonized polymer dots RCBCPDs with red light are developed, and the carbonized polymer dots are simple in preparation method, excellent in fluorescent property and wide in application prospect. When the RCBCPDs obtained by the invention are applied to cell imaging and in-vivo imaging, the RCBCPDs show interference resistance in cells and mice, and have the advantages of high selectivity, high sensitivity and the like.
Owner:XIHUA UNIV
Stable co-formulation of benzoylurea with pyrethroids
PendingUS20210329916A1Low toxicityBiocideDead animal preservationChrysanthemum cinerariifoliumPerylene derivatives
The present invention provides a microencapsulated formulation comprising a pyrethroid insecticide solubilized in vegetable oil or derivatives thereof, the solubilized pyrethroid insecticide being encapsulated in a capsule having a polymeric shell wall. The invention also provides a process of preparing said microencapsulated formulation. The invention further provides a co-formulation comprises microencapsulated formulation of pyrethroid and a suspension concentrate comprising benzoylurea insecticide.
Owner:UNITED PHOSPHORUS LTD
Pesticidal and acaricidal composition and application thereof
ActiveCN105265460AReduce pollutionGood synergyBiocideDead animal preservationChemical compositionTetranychus cinnabarinus
The invention discloses a pesticidal and acaricidal composition and application thereof, and relates to the technical field of pesticide mixing. The pesticidal and acaricidal composition is prepared from an effective component A and an effective component B, the effective component A is amitraz, the effective component B is benzoylurea insecticide, and the weight ratio of effective component A to the effective component B is 1:60-70:1. The mixed preparation is reasonable in matching, has a remarkable synergistic effect, particularly has an excellent effect on lepidoptera pests such as plutella xylostella and prodenia litura as well as agricultural acarid such as tetranychus cinnabarinus and tetranychus urticae, can effectively reduce application amount of all single agents, achieves the purposes of delaying pesticide resistance and reducing cost and has obvious application and popularization value.
Owner:LIER CROPSCIENCE CO LTD
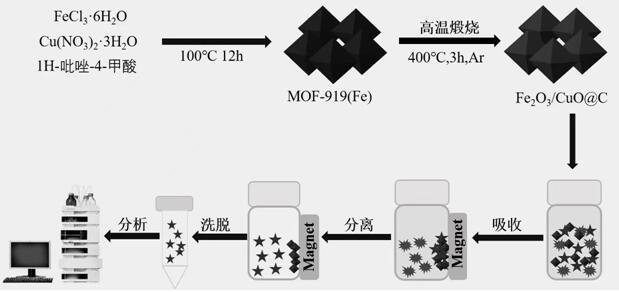
Preparation and application of bimetallic oxide magnetic carbon material derived from metal organic framework
ActiveCN114713196AImprove featuresStrong magnetic responseIon-exchange process apparatusOther chemical processesMagnetic carbonMetal-organic framework
The invention provides preparation and application of a bimetallic oxide magnetic carbon material derived from a metal organic framework. The bimetallic oxide magnetic carbon material is formed by calcining and carbonizing a bimetallic organic framework MOF-919 (Fe) material under a high-temperature condition, and is applied to enrichment and separation of a target object in a complex system sample. The preparation method comprises the following steps: synthesizing a bimetallic organic framework MOF-919 (Fe) material by taking Fe and Cu as metal nodes and 4-pyrazolecarboxylic acid as a ligand, calcining the material at high temperature under the protection of inert gas, and directly carbonizing to obtain the bimetallic oxide magnetic carbon material Fe2O3 / CuO-C. Magnetic particles and a carbon-based precursor do not need to be added in the preparation process, and the prepared magnetic carbon material has relatively high specific surface area, good porosity, excellent magnetic performance and rich adsorption sites, and can be applied to magnetic solid-phase extraction as an adsorption material to efficiently enrich and separate benzoylurea insecticides.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Novel benzoylurea compound and use thereof
The present invention relates to a new type benzolyurea compound and its application. It is characterized by that it uses compound isocyanate and compound chlorothalonil derivative, and makes them produce reaction to prepare the invented product with higher insecticidal and ovicidal action. Said invention also provides its structural formula.
Owner:YUNNAN UNIV
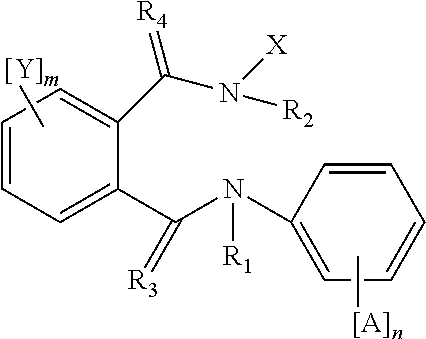
Active compound combinations having insecticidal properties
The present invention relates to novel active compound combinations comprising at least one known compound of the formula (I)in which X, R1 to R4, A, n, Y, and m are as defined in the description, and at least one further known active compound from the class of the of tetronic and tetramic acid derivatives, diacylhydrazines, benzoylureas and further classes, which combinations are highly suitable for controlling animal pests such as insects and unwanted acarids.
Owner:BAYER CROPSCIENCE AG
Chlorpyrifos-methyl and benzoylurea pesticide containing composition, as well as preparation and application thereof
InactiveCN106614712ASignificant synergyRelieve pressureBiocideDead animal preservationNovaluronBuprofezin
The invention provides a chlorpyrifos-methyl and benzoylurea pesticide containing composition, which contains chlorpyrifos-methyl and a benzoylurea pesticide, wherein the benzoylurea pesticide is one or more selected from novaluron, teflubenzuron, diflubenzuron, triflumuron, hexaflumuron, flufenoxuron, buprofezin, flucycloxuron, chlorbenzuron and lufenuron; the mass ratio of the chlorpyrifos-methyl to the benzoylurea pesticide is (1 to 80) to (80 to 1), preferably (1 to 40) to (40 to 1), more preferably (1 to 20) to (20 to 1) and most preferably (1 to 10) to (10 to 1). The composition has remarkable synergistic effects, the dosage can be reduced, and the drug cost can be greatly reduced.
Owner:ZHEJIANG XINNONG CHEM CO LTD
Preparation method of benzoylurea anthelmintic
PendingCN114805140AAvoid it happening againPost-processing is simpleUrea derivatives preparationBiocideAnthelmintic drugDistillation
The invention discloses a preparation method of a benzoylurea anthelmintic. The preparation method comprises the following steps: by taking 2, 5-dichloro-4-(1, 1, 2, 3, 3, 3-hexafluoropropoxy) nitrobenzene and 2, 6-difluorobenzamide as initial raw materials, reducing the 2, 5-dichloro-4-(1, 1, 2, 3, 3, 3-hexafluoropropoxy) nitrobenzene through hydrazine hydrate, reacting the 2, 6-difluorobenzamide with oxalyl chloride to generate isocyanate, and finally performing amidation reaction to obtain the lufenuron. The invention provides the preparation method of the veterinary drug lufenuron, which is green, environment-friendly, mild in reaction, less in side reaction, short in synthetic route, simple and convenient to operate and high in purity, the same green solvent is adopted, high-toxicity dichloroethane is not used as a solvent, post-treatment is simplified, and oxidation byproducts are prevented from being generated in the high-temperature distillation process of the reduzate. A mild hydrazine hydrate reduction method is adopted, so that propoxy bond breakage and dechlorination impurities generated by catalytic hydrogenation reduction are avoided, and then impurities B and C are generated in a finished product. The purity of the prepared lufenuron impurity can reach 99.8% or above, and the yield reaches 86.0% or above.
Owner:CHANGZHOU YABANG QH PHARMACHEM +1
Composition containing fluchlordiniliprole and benzoylurea insecticide
InactiveCN112970763AMeet safety requirementsImprove securityBiocideAnimal repellantsCnaphalocrocis medinalisSpodoptera
The invention belongs to the field of pesticides, and particularly relates to a composition containing fluchlordiniliprole and a benzoylurea insecticide. The composition is composed of an active component, a filling material and an auxiliary agent, the active component is composed of fluchlordiniliprole and a benzoylurea insecticide, and the benzoylurea insecticide is hexaflumuron. The composition is mainly used for preventing and treating lepidoptera pests such as plutella xylostella, beet armyworm, prodenia litura and cnaphalocrocis medinalis. The composition has many action mechanisms and sites, and can effectively delay the occurrence and development of pest resistance to drugs; the preparation process is simple, low in cost and remarkable in economic benefit.
Owner:HAILIR PESTICIDES & CHEM GRP
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com