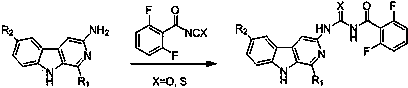
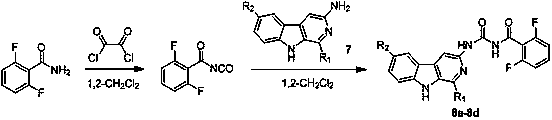
Banisterine benzoyl urea compounds and preparation method and application thereof
A technology for benzoyl urea and camel ketone, applied in the field of pesticides, can solve the problems such as the research and synthesis reports of benzoyl urea compounds of camel pine, and achieves simple structure, simple synthesis process and good inhibitory activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
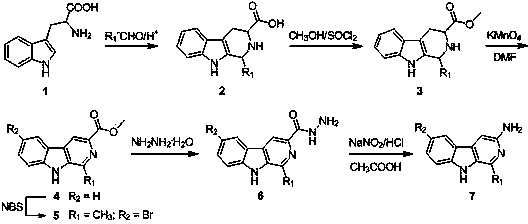
[0048] Example 1: Synthesis of 1-methyl-β-carboline-3-ammonia (6a)
[0049] (Refer to literature method (Lin G.W., et al. Synthetic Communications , 2012, 42 , 1895-1910, namely reaction formula II).
[0050] 1. Step A: 1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid ( 2 )Synthesis
[0051] Weigh 20.4g of L-tryptophan (0.1mol) into a three-necked flask equipped with an electric heating mantle and electric stirrer, add 70-80 mL of glacial acetic acid and 5mL of 40% acetaldehyde (0.12mol) under stirring, After stirring for 15 minutes, heat up to 80-100°C and react for 10 hours. TLC traces until the tryptophan disappears, stops heating, cools to room temperature and precipitates form, filters, and concentrates the filtrate under reduced pressure to remove excess acetic acid and water to obtain a large amount of shallow Brown flakes were washed with water to obtain a white product with a yield of 85%.
[0052] 2. Step B: methyl 1-methyl-1,2,3,4-tetrahydro-β-carbo...
Embodiment 2
[0060] Example 2: Synthesis of 1-phenyl-β-carboline-3-ammonia (7b)
[0061] Operation is the same as in Example 1, only in step A replace acetaldehyde with benzaldehyde. A yellow-green powder was finally obtained with a yield of 87%.
Embodiment 3
[0062] Example 3: Synthesis of 1-p-chlorophenyl-β-carboline-3-ammonia (7c)
[0063] Operation is the same as in Example 1, only in step A, acetaldehyde is replaced with p-chlorobenzaldehyde. Yellow-green flaky crystals were finally obtained with a yield of 79%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com