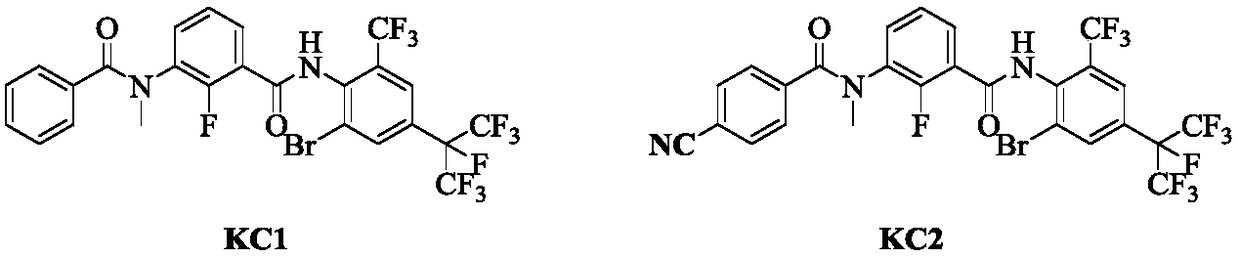
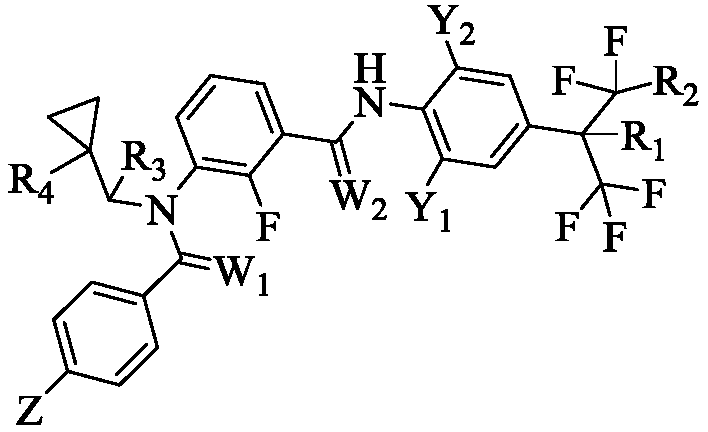
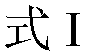M-diamide compound and preparation method and application thereof
A technology of isodiamide and compound, which is applied in the field of compound and its preparation in which the 3-position of isodiamide is substituted by N-cyclopropylmethyl derivatives, can solve the problems of poor quick-acting property and poor insecticidal effect, and achieve High insecticidal activity, fast onset, and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] N-[2-Bromo-4-(1,1,1,2,3,3,3-heptafluoroprop-2-yl)-6-(trifluoromethyl)phenyl]-3-[N- (Cyclopropylmethyl)-benzamido]-2-fluorobenzamide (compound No. 4):
[0156] (1) Synthesis of 2-fluoro-[3-(cyclopropylmethyl)amino]benzoic acid methyl ester
[0157]
[0158] Add methyl 2-fluoro-3-aminobenzoate (20g, 118.23mmol), bromomethylcyclopropane (20.75g, 153.70mmol), potassium carbonate (21.24g, 153.70mmol), N, N-dimethylformamide (200 mL) was stirred under reflux for 16 h. When TLC monitored that the reaction did not proceed, the heating was turned off to terminate the reaction. After the reaction solution was cooled to room temperature, water (200 mL) was added to the reaction solution, extracted with ethyl acetate (100 mL), the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was washed with Purified by column chromatography (eluent is petroleum ether: ethyl acetate = 10:1), the product ...
Embodiment 2
[0171] N-[2-Bromo-4-(1,1,1,2,3,3,3-heptafluoroprop-2-yl)-6-(trifluoromethyl)phenyl]-3-[N- (Cyclopropylmethyl)-4-cyanobenzamido]-2-fluorobenzamide (compound number 23):
[0172] (1) Synthesis of methyl 2-fluoro-3-[N-(cyclopropylmethyl)-4-cyanobenzamido]benzoate
[0173]
[0174] Add 4-cyanobenzoic acid (0.80g, 5.38mmol), toluene (6mL), and thionyl chloride (3.2g, 26.9mmol) to the reaction flask successively, stir the reaction under reflux for 2h, and concentrate the toluene under reduced pressure , the concentrated residue was dissolved in THF (3 mL) for use. Dissolve 2-fluoro-[3-(cyclopropylmethyl)amino]methyl benzoate (1.0g, 4.48mmol) in tetrahydrofuran (6mL), add triethylamine (0.74g, 5.38mmol), dropwise add 4 -The tetrahydrofuran solution of cyanobenzoyl chloride was stirred at room temperature for 4h. When TLC monitors that the reaction no longer proceeds, the reaction is terminated. Water (20 mL) was added to the reaction solution, extracted with ethyl acetate (20 ...
Embodiment 3
[0184] N-[2-Bromo-4-(1,1,1,2,3,3,3-heptafluoroprop-2-yl)-6-(trifluoromethyl)phenyl]-3-[N- Preparation of (cyclopropylmethyl)-4-(trifluoromethyl)benzamido]-2-fluorobenzamide (compound number 37):
[0185]
[0186] (1) Add 2-fluoro-3-[N-(cyclopropylmethyl)-4-trifluoromethylbenzamide]benzoic acid (0.45g, 1.12mmol) and toluene (6mL) successively to the reaction flask, Thionyl chloride (0.67 g, 5.60 mmol) was stirred under reflux for 2 h, the toluene was concentrated under reduced pressure, and the concentrated residue was dissolved in tetrahydrofuran (3 mL) for further use.
[0187] (2) Dissolve 2-bromo-6-trifluoromethyl-4-heptafluoroisopropylaniline (0.46g, 1.12mmol) in tetrahydrofuran (4mL), and add lithium diisopropylamide dropwise at -70°C (0.70mL, 1.42mmol), after 5min, add the tetrahydrofuran solution to be used dropwise, stir at -70°C for 30min, warm to room temperature and continue stirring for 30min. When TLC monitors that the reaction no longer proceeds, the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com