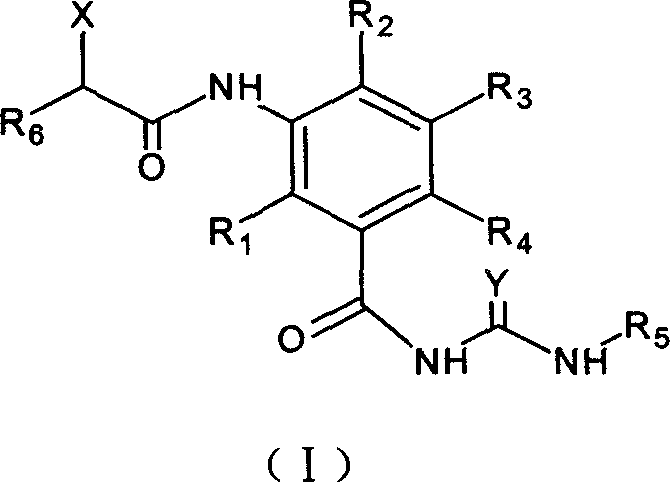
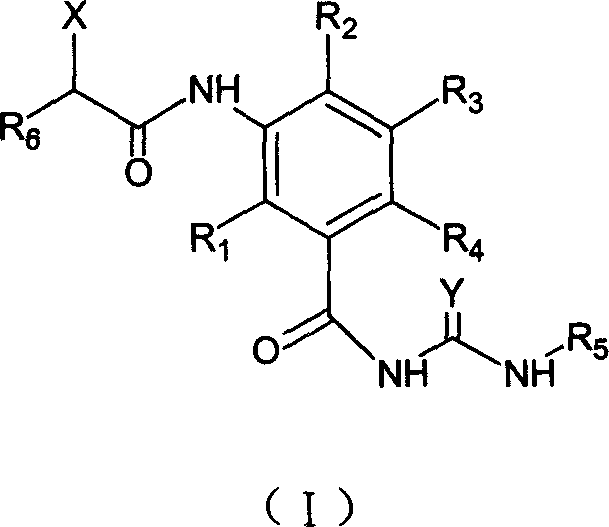
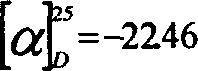3-amido substituted nenzoyl urea compound, and its antitumor action
A compound and anti-tumor drug technology, applied in the field of microtubule and microfilament inhibitor benzoyl urea small molecule derivatives, can solve the problems such as the anti-tumor effect of compounds that have not yet been seen, and achieve the effect of strong cell killing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] : Preparation of enantiomers (-)-JIMB01 and (+)-JIMB01
[0017] Preparation of chiral side chains: Dissolve 0.46g (0.30mmol) of 2-bromopropionic acid in 10ml of anhydrous dichloromethane, add 0.54g (0.45mmol) of thionyl chloride, heat to reflux for 2h, evaporate the solvent and excess Thionyl chloride can be obtained.
[0018] 13.7g (0.1mol) of 3-aminobenzoic acid was dissolved in 100ml of ethyl acetate (EA), and 15.53ml (0.11mol) of trifluoroacetic anhydride was added dropwise, and heated to reflux for 1h. The solid was filtered, washed thoroughly with EA, and dried to constant weight to obtain a white solid to obtain 3-(N-trifluoroacetylamino)benzoic acid (A-1), yield 86% (20.0 g), melting point: 285°C.
[0019] Dissolve 4.66g (0.02mol) of A-1 in 50ml of EA, add 15ml of thionyl chloride (SOCl2) dropwise, and heat to reflux until the reaction solution becomes a clear yellow liquid (1h). The solvent and the remaining SOCl2 were evaporated, and recrystallized with cycl...
Embodiment 2
[0029] : Preparation of 4-chlorobenzoylurea compounds F-1, F-2, F-3, F-4
[0030] Take 17.20g (0.1mol) of 4-chloro-3-aminobenzoic acid and dissolve it in 100ml of ethyl acetate (EA), add dropwise 15.53ml (0.11mol) of trifluoroacetic anhydride, and heat to reflux for 1h. The solid was filtered, washed thoroughly with EA, dried to constant weight to obtain a white solid, and 4-chloro-3-(N-trifluoroacetylamino)benzoic acid (B-1) was obtained in a yield of 91.2% (24.4g). Melting point: 158°C-160C.
[0031] Dissolve 5.35g (0.02mol) (B-1) in 50ml of EA, add 15ml of SOCl2 dropwise, and heat to reflux until the reaction solution becomes a clear yellow liquid (1h). Evaporate solvent and remaining SOCl2, and carry out recrystallization with cyclohexane to obtain pale yellow crystals, obtain 4-chloro-3-(N-trifluoroacetylamino) benzoyl chloride (B-2), yield 85% ( 4.86g), melting point: 98°C-100°C.
[0032] Dissolve 5.72g (0.02mol) (B-2) in 50ml of toluene, add 6.00g (0.1mol) of urea, s...
Embodiment 4
[0077] : Synthesis of 4-methylbenzoylurea compounds F-21, F-22, F-23, F-24
[0078] Take 15.1g (0.1mol) of 4-methyl-3-aminobenzoic acid and dissolve it in 100ml of ethyl acetate (EA), add dropwise 15.53ml (0.11mol) of trifluoroacetic anhydride, and heat to reflux for 1h. The solid was filtered, washed thoroughly with EA, and dried to constant weight to obtain a white solid to obtain 4-methyl-3-(N-trifluoroacetylamino)benzoic acid (D-1). Yield: 92.3% (22.81g), melting point: 174°C-176°C.
[0079] Dissolve (D-1) 4.94g (0.02mol) in 50ml EA, add dropwise 15ml SOCl2, and heat to reflux until the reaction solution becomes a clear yellow liquid (1h). The solvent and the remaining SOCl2 were evaporated, and recrystallized with cyclohexane to obtain pale yellow crystals to obtain 4-methyl-3-(N-trifluoroacetylamino)benzoyl chloride (D-2). Yield: 84.4% (4.48g), melting point: 108°C-110°C.
[0080] Dissolve 5.30g (0.02mol) of (D-2) in 50ml of toluene, add 6.00g (0.1mol) of urea, slowly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com