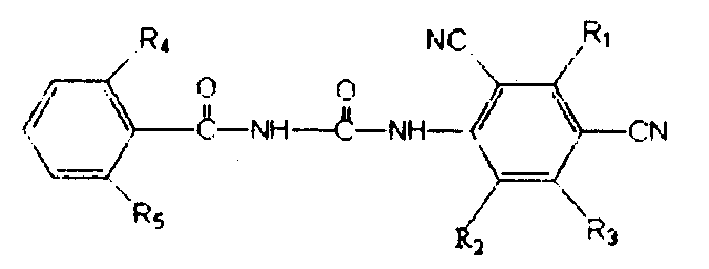
Novel benzoylurea compound and use thereof
A technology of benzoyl urea and compounds, which is applied in new benzoyl urea compounds and their application fields, and can solve problems such as difficult synthesis, complex structure of substituted phenyl groups, and increased overall synthesis difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] N-(2-chlorobenzoyl)-N'-(2,4-dinitrile-3,5,6-trifluorophenyl)urea Weigh 0.1 mole of just prepared benzoyl isocyanate II-1, and use Dry 1,2-dichloroethane to dissolve and dilute it in a flask equipped with a stirrer; weigh another 0.11 mole of aniline derivative IV-3, and also use dry 1,2-dichloroethane to make it fully Dissolved to make a solution, then slowly drop it into the above-mentioned ice-cooled reaction flask with stirring, and a white solid was precipitated immediately. After the exothermic reaction was completed, the reaction was continued for 24 hours, and the obtained solid was suction-filtered. Recrystallization gave 37 grams of white needle crystal N-(2-chlorobenzoyl)-N'-(2,4-dinitrile-3,5,6-trifluorophenyl)urea (I-3), producing The rate is 92%.
Embodiment 2
[0052] N-(2,6-difluorobenzoyl)-N'-(2,4-dinitrile-3,5,6-trifluorophenyl)urea weighed 0.1 mole of freshly prepared benzoyl isocyanate II- 2. Use dry 1,2-dichloroethane to dissolve and dilute it in a flask equipped with a stirrer; weigh 0.12 moles of aniline derivative IV-3, and also use dry 1,2-dichloroethane It was fully dissolved to make a solution, and then slowly dripped into the above-mentioned ice-cooled reaction flask under stirring, and a white solid was precipitated immediately. After the heat release was completed, the reaction was continued for 24 hours, and the obtained solid was filtered with suction. Recrystallization gave white needle crystal N-(2,6-difluorobenzoyl)-N'-(2,4-dinitrile-3,5,6-trifluorophenyl)urea (I-4)36 g, yield 94%.
Embodiment 3
[0054] N-(2,6-difluorobenzoyl)-N'-(2,4-dinitrile-3,6-difluoro-5-aminophenyl)urea Weigh 0.1 mole of freshly prepared benzoyl isocyanate II-2, use dry 1,2-dichloroethane to dissolve and dilute it in a flask equipped with a stirrer; take another 0.13 mole of aniline derivative IV-4, also use dry 1,2-dichloro Ethane was fully dissolved to make a solution, and then slowly dripped into the above-mentioned ice-cooled reaction flask under stirring, and a white solid was precipitated immediately. After the heat release was completed, the reaction was continued for 24 hours, and the obtained solid was filtered with suction. Recrystallization gave white needle crystal N-(2,6-difluorobenzoyl)-N'-(2,4-dinitrile-3,6-difluoro-5-aminophenyl)urea (I-7 ) 35 grams, yield 90%.
[0055] In the following, the insecticidal effect of the benzoylurea derivative preparation will be illustrated by taking the indoor insecticidal effect of the preparation containing the benzoylurea derivative.
[0056] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com