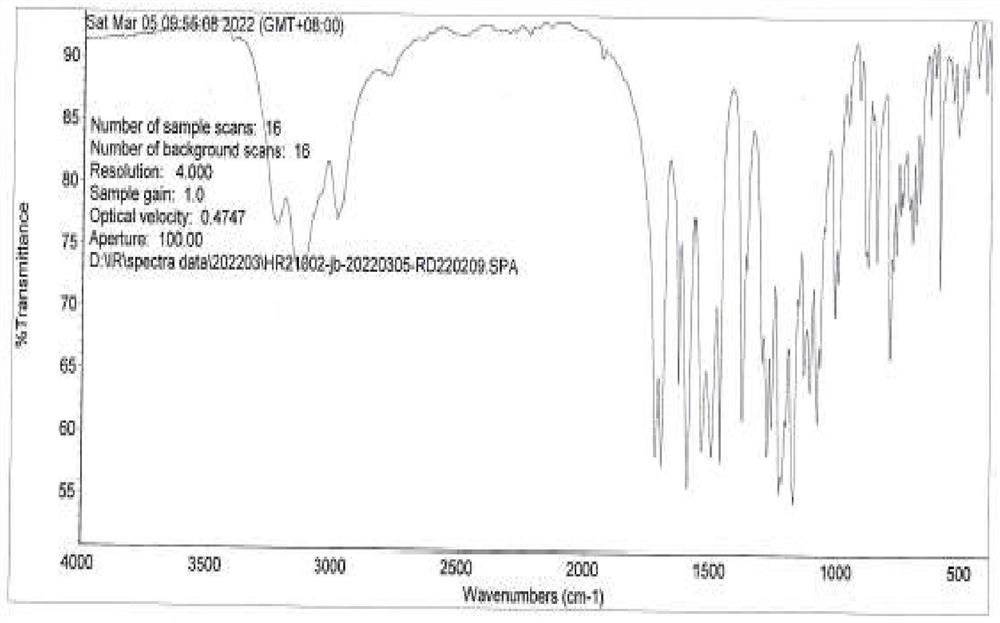
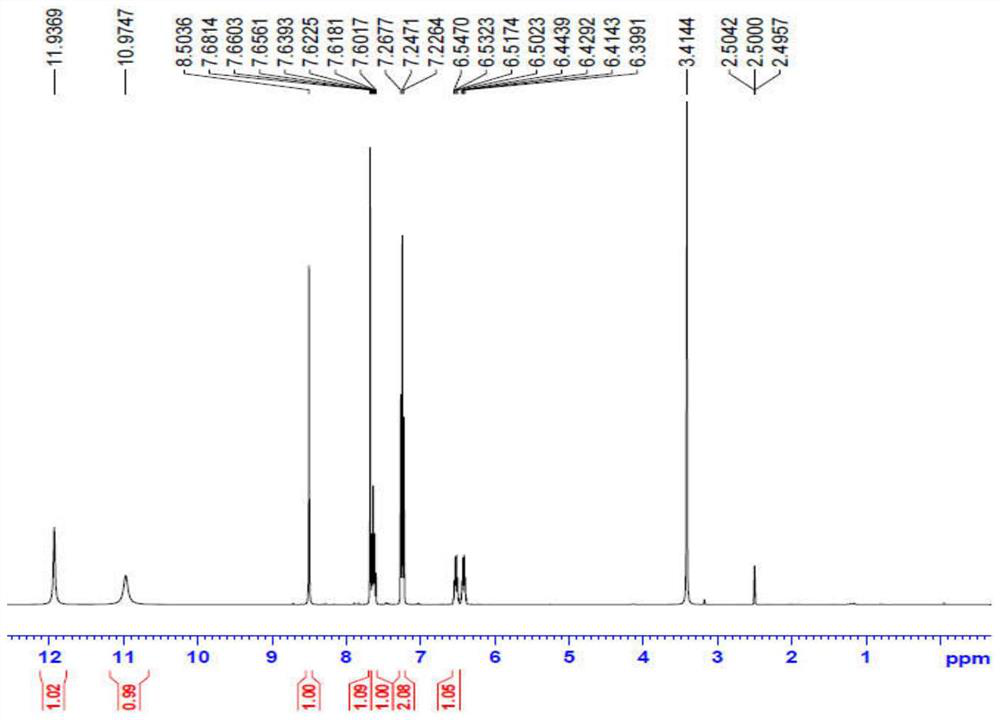
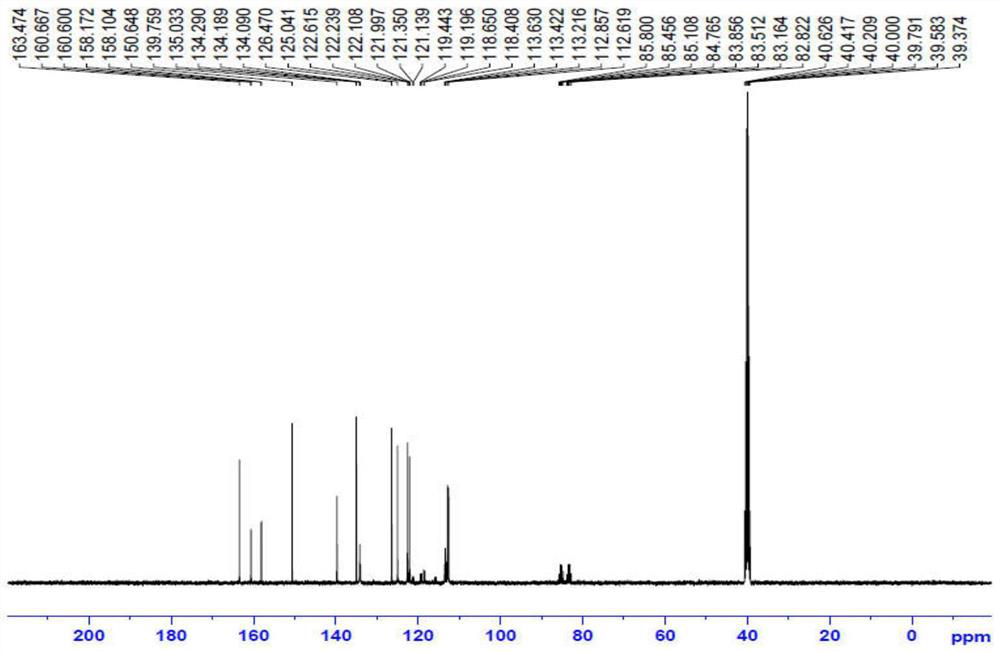
Preparation method of benzoylurea anthelmintic
A technology for benzoyl urea and anthelmintic medicine, which is applied in the field of preparation of benzoyl urea anthelmintic medicine, can solve problems such as high toxicity, and achieve the effects of simplifying post-processing and avoiding oxidation by-products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] To a 500ml four-necked flask, add 300ml of p-xylene, 2,5-dichloro-4-(1,1,2,3,3,3-hexafluoropropoxy) nitrobenzene is 35.8g (0.1mol) , 0.2g of ferric oxyhydroxide and 0.8g of activated carbon, start stirring, heat up to 65 ° C, add 10.5 g of hydrazine hydrate dropwise, control the temperature to 65-69 ° C, and drop the time for 0.5 hours. To 25-30 ℃, stand, separate the lower water layer, wash the organic layer with 1 times the amount of water, separate the lower water layer to obtain the reduced product 2,5-dichloro-4-(1,1,2,3 , 3,3-hexafluoropropoxy) aniline in p-xylene.
[0042]16.5g (0.105mol) of 2,6-difluorobenzamide and 66g of p-xylene were added to the 500ml reaction flask, the temperature was raised to 25°C with stirring, 19.0g (0.15mol) of oxalyl chloride was slowly added dropwise, and the reaction temperature was controlled to 35°C , the dropping time was 1 hour, and the temperature was raised to 110 ° C after the dropping was completed for 1 hour. After the re...
Embodiment 2
[0048] To a 500ml four-necked flask, add 200ml of o-xylene, 2,5-dichloro-4-(1,1,2,3,3,3-hexafluoropropoxy) nitrobenzene is 35.8g (0.1mol) , 0.7g of ferric chloride and 1.0g of activated carbon, turn on stirring, heat up to 65 ° C, dropwise add 18.5 g of hydrazine hydrate, control the temperature to be 65-69 ° C, and drop the time for 1 hour, after dropping the insulation reaction for 1 hour, cooling down To 25-30 ℃, stand, separate the lower water layer, wash the organic layer with 1 times the amount of water, separate the lower water layer to obtain the reduced product 2,5-dichloro-4-(1,1,2,3 , 3,3-hexafluoropropoxy) aniline solution in o-xylene.
[0049] Add 16.5g (0.105mol) of 2,6-difluorobenzamide and 120g of o-xylene to the 500ml reaction flask, stir and heat up to 25°C, slowly add 21.5g (0.17mol) of oxalyl chloride dropwise, and control the reaction temperature to 25°C , the dropping time is 1 hour, and the temperature is raised to 112° C. after the dropwise addition is...
Embodiment 3
[0054] To a 500ml four-necked flask, add 320ml of toluene, 2,5-dichloro-4-(1,1,2,3,3,3-hexafluoropropoxy) nitrobenzene is 35.8g (0.1mol), hydrogen 0.5g of bismuth oxide and 1.2g of activated carbon, turn on stirring, heat up to 65°C, add 12.5g of hydrazine hydrate dropwise, control the temperature to be 65-69°C, add dropwise time for 0.5 hour, finish the dripping for 3 hours, and cool down to 25 -30℃, stand still, separate the lower water layer, wash the organic layer with 1 times the amount of water, separate the lower water layer to obtain the reduced product 2,5-dichloro-4-(1,1,2,3,3 , 3-hexafluoropropoxy) aniline in toluene.
[0055] 17.2g (0.11mol) of 2,6-difluorobenzamide and 70g of toluene were added to the 500ml reaction flask, the temperature was raised to 25°C with stirring, and 17.3g (0.14mol) of oxalyl chloride was slowly added dropwise, and the reaction temperature was controlled at 30°C. Add time for 1 hour, be incubated for 1 hour after the dropwise addition is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com