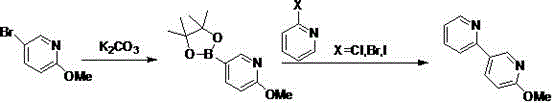
2-methoxy-5-(pyridine-2-yl)pyridine synthesis method
A methoxypyridine, methoxyl technology, applied in the field of medicinal chemistry, can solve the problem of high cost, achieve the effects of low cost, convenient large-scale production and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0020] Add 1.57 gK2CO3 and 6.25 ml DMF to a 100ml three-necked flask equipped with a thermometer, a constant pressure dropping funnel, and magnetic stirring. Stir, add 1.41 ml 5-bromo-2-methoxypyridine slowly, and the addition is complete. Slowly add 2.07 g of nadiboron, stirring, and react for 2 hours. Add 1.7 g of 2-chloropyridine and stir to react for 3 hours, dilute with water, stir for 20 min, and separate; the aqueous phase is extracted with ethyl acetate, the organic phases are combined, washed twice with saturated brine, and concentrated under reduced pressure to obtain the middle of perampanel Body yellow oil, purity 83%, yield 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com